当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic basis for the activation of human cyclooxygenase-2 rather than cyclooxygenase-1 by nitric oxide†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7ob02992f Jie Qiao 1, 2, 3, 4, 5 , Lixin Ma 1, 2, 3, 4, 5 , Justine Roth 6, 7, 8, 9 , Yamin Li 4, 5, 10, 11 , Yi Liu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7ob02992f Jie Qiao 1, 2, 3, 4, 5 , Lixin Ma 1, 2, 3, 4, 5 , Justine Roth 6, 7, 8, 9 , Yamin Li 4, 5, 10, 11 , Yi Liu 1, 2, 3, 4, 5
Affiliation
|
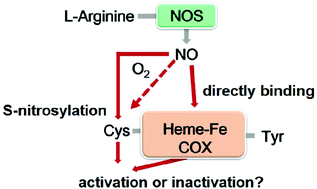
Numerous studies have shown that nitric oxide (NO) interacts with human cyclooxygenase (COX); however, conflicting results exist with respect to their interactions. Herein, recombinant human COX-1 and COX-2 were prepared and treated with NO donors individually under anaerobic and aerobic conditions. The S-nitrosylation detection and subsequent kinetic investigations into the arachidonic acid (AA) oxidation of COX enzymes indicate that NO S-nitrosylates both COX-1 and COX-2 in an oxygen-dependent manner, but enhances only the dioxygenase activity of COX-2. The solution viscosity, deuterium kinetic isotope effect (KIE), and oxygen-18 KIE experiments further demonstrate that NO activates COX-2 by altering the protein conformation to stimulate substrate association/product release and by accelerating the rate of hydrogen abstraction from AA by catalytic tyrosine radicals. These novel findings provide useful information for designing new drugs with less cardiotoxic effects that can block the interaction between NO and COX.
中文翻译:

一氧化氮活化人环氧合酶2而不是环氧合酶1的动力学基础†
大量研究表明,一氧化氮(NO)与人环氧合酶(COX)相互作用。但是,在它们的相互作用方面存在矛盾的结果。在此,制备重组人COX-1和COX-2,并在厌氧和有氧条件下分别用NO供体处理。该小号-nitrosylation检测和随后的动力学调查COX酶的花生四烯酸(AA)氧化表明NO小号-亚硝酰基以氧依赖的方式使COX-1和COX-2均氧依赖性,但仅增强COX-2的双加氧酶活性。溶液粘度,氘动力学同位素效应(KIE)和氧18 KIE实验进一步证明,NO可以通过改变蛋白质构象以刺激底物缔合/产物释放,并通过催化从AA提取氢的速率来激活COX-2。酪氨酸基团。这些新颖的发现为设计具有更低心脏毒性作用的新药物提供了有用的信息,这些药物可以阻止NO和COX之间的相互作用。
更新日期:2017-12-21
中文翻译:

一氧化氮活化人环氧合酶2而不是环氧合酶1的动力学基础†
大量研究表明,一氧化氮(NO)与人环氧合酶(COX)相互作用。但是,在它们的相互作用方面存在矛盾的结果。在此,制备重组人COX-1和COX-2,并在厌氧和有氧条件下分别用NO供体处理。该小号-nitrosylation检测和随后的动力学调查COX酶的花生四烯酸(AA)氧化表明NO小号-亚硝酰基以氧依赖的方式使COX-1和COX-2均氧依赖性,但仅增强COX-2的双加氧酶活性。溶液粘度,氘动力学同位素效应(KIE)和氧18 KIE实验进一步证明,NO可以通过改变蛋白质构象以刺激底物缔合/产物释放,并通过催化从AA提取氢的速率来激活COX-2。酪氨酸基团。这些新颖的发现为设计具有更低心脏毒性作用的新药物提供了有用的信息,这些药物可以阻止NO和COX之间的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号