当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed enantioselective C(sp2)–H arylation of ferrocenyl ketones enabled by a chiral transient directing group
Chemical Communications ( IF 4.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7cc09273c Jiancong Xu 1, 2, 3, 4, 5 , Yang Liu 1, 2, 3, 4, 5 , Jinling Zhang 1, 2, 3, 4, 5 , Xiaohua Xu 1, 2, 3, 4, 5 , Zhong Jin 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7cc09273c Jiancong Xu 1, 2, 3, 4, 5 , Yang Liu 1, 2, 3, 4, 5 , Jinling Zhang 1, 2, 3, 4, 5 , Xiaohua Xu 1, 2, 3, 4, 5 , Zhong Jin 1, 2, 3, 4, 5
Affiliation
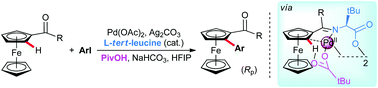
|
Palladium-catalyzed enantioselective C(sp2)–H activation of ferrocenyl ketones is achieved through utilizing catalytic, inexpensive L-tert-leucine as a chiral transient directing group. The transformation allows rapid access to ferrocene scaffolds simultaneously possessing planar- and stereogenic central chirality, widely applied in the ferrocene-based chiral ligand families.
中文翻译:

钯催化的二茂铁基酮的对映体选择性C(sp 2)–H芳基化由手性瞬态导向基团实现
钯-催化的对映选择性C(SP 2)-H的二茂铁酮的活化是通过利用催化的,廉价的实现大号-叔-亮氨酸作为手性瞬态引导组。该转化允许快速获得同时具有平面和立体中心手性的二茂铁支架,广泛应用于基于二茂铁的手性配体家族。
更新日期:2018-01-16
中文翻译:

钯催化的二茂铁基酮的对映体选择性C(sp 2)–H芳基化由手性瞬态导向基团实现
钯-催化的对映选择性C(SP 2)-H的二茂铁酮的活化是通过利用催化的,廉价的实现大号-叔-亮氨酸作为手性瞬态引导组。该转化允许快速获得同时具有平面和立体中心手性的二茂铁支架,广泛应用于基于二茂铁的手性配体家族。



























 京公网安备 11010802027423号
京公网安备 11010802027423号