当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenated Blue Titania for Efficient Solar to Chemical Conversions: Preparation, Characterization, and Reaction Mechanism of CO2 Reduction
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acscatal.7b03473 Guoheng Yin 1, 2 , Xieyi Huang 1 , Tianyuan Chen 3 , Wei Zhao 1 , Qingyuan Bi 1 , Jing Xu 3 , Yifan Han 3 , Fuqiang Huang 1, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acscatal.7b03473 Guoheng Yin 1, 2 , Xieyi Huang 1 , Tianyuan Chen 3 , Wei Zhao 1 , Qingyuan Bi 1 , Jing Xu 3 , Yifan Han 3 , Fuqiang Huang 1, 4
Affiliation
|
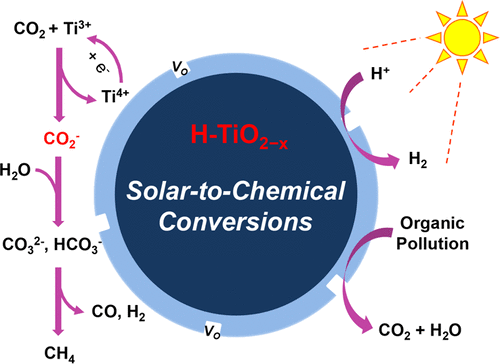
Here we report a facile low-temperature solvothermal method by using Li-dissolved ethanediamine to prepare uniform hydrogenated blue H-TiO2–x with wide spectrum response. H-TiO2–x possesses a distinct crystalline core–amorphous shell structure (TiO2@TiO2–x) with numerous oxygen vacancies and doped H in the amorphous shell. Efficient solar to chemical energy conversions, likely photocatalytic reduction of CO2, degradation of contaminants, and H2 generation from water splitting can be achieved over this blue titania. Notably, the optimized H-TiO2–x(200) shows high activity of CH4 formation at a rate of 16.2 μmol g–1 h–1 and a selectivity of 79% under full solar irradiation. The kinetic isotope effects measurements reveal that the cleavage of the C═O bond from CO2 rather than the O–H bond from H2O is the rate-determining step in CH4 formation. Meanwhile, in situ diffuse reflectance infrared Fourier transform spectroscopy shows the existence of the key intermediate CO2– species. The formation of intermediate CO2– indicates that the defective surface of H-TiO2–x can efficiently accelerate the adsorption and chemical activation of the extremely stable CO2 molecule, which makes the single-electron reduction of CO2 to CO2– easier.
中文翻译:

氢化蓝二氧化钛可实现高效的太阳到化学转化:CO 2还原的制备,表征和反应机理
在这里,我们报告了一种简便的低温溶剂热方法,该方法通过使用溶解锂的乙二胺制备具有宽光谱响应的均匀氢化蓝色H-TiO 2 – x。H-TiO 2 – x具有独特的晶体核–无定形壳结构(TiO 2 @TiO 2– x),具有许多氧空位,并且在无定形壳中掺杂了H。可以在该蓝色二氧化钛上实现高效的太阳能到化学能的转换,可能的光催化还原CO 2,污染物的降解以及水分解产生的H 2。值得注意的是,经过优化的H-TiO 2 – x(200)具有较高的CH 4活性。在完全阳光照射下,其形成速率为16.2μmolg –1 h –1,选择性为79%。动力学同位素效应的测量表明,CO 2中的C═O键而不是H 2 O中的O–H键断裂是决定CH 4形成的速率步骤。同时,在原位漫反射傅里叶变换红外光谱示出了关键中间体CO的存在2 -物种。中间CO 2 –的形成表明H-TiO 2 – x的缺陷表面可以有效地加速极其稳定的CO 2的吸附和化学活化。分子,这使得CO的单电子还原2至CO 2 -更容易。
更新日期:2018-01-08
中文翻译:

氢化蓝二氧化钛可实现高效的太阳到化学转化:CO 2还原的制备,表征和反应机理
在这里,我们报告了一种简便的低温溶剂热方法,该方法通过使用溶解锂的乙二胺制备具有宽光谱响应的均匀氢化蓝色H-TiO 2 – x。H-TiO 2 – x具有独特的晶体核–无定形壳结构(TiO 2 @TiO 2– x),具有许多氧空位,并且在无定形壳中掺杂了H。可以在该蓝色二氧化钛上实现高效的太阳能到化学能的转换,可能的光催化还原CO 2,污染物的降解以及水分解产生的H 2。值得注意的是,经过优化的H-TiO 2 – x(200)具有较高的CH 4活性。在完全阳光照射下,其形成速率为16.2μmolg –1 h –1,选择性为79%。动力学同位素效应的测量表明,CO 2中的C═O键而不是H 2 O中的O–H键断裂是决定CH 4形成的速率步骤。同时,在原位漫反射傅里叶变换红外光谱示出了关键中间体CO的存在2 -物种。中间CO 2 –的形成表明H-TiO 2 – x的缺陷表面可以有效地加速极其稳定的CO 2的吸附和化学活化。分子,这使得CO的单电子还原2至CO 2 -更容易。











































 京公网安备 11010802027423号
京公网安备 11010802027423号