当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Ionic Liquids on the Structure and Dynamics of Collagen
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acs.jpcb.7b09626 Aafiya Tarannum 1 , Alina Adams 2 , Bernhard Blümich 2 , Nishter Nishad Fathima 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acs.jpcb.7b09626 Aafiya Tarannum 1 , Alina Adams 2 , Bernhard Blümich 2 , Nishter Nishad Fathima 1
Affiliation
|
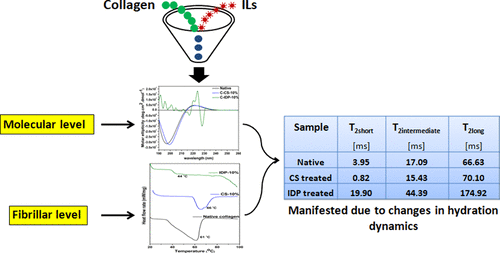
The changes in the structure and dynamics of collagen treated with two different classes of ionic liquids, bis-choline sulfate (CS) and 1-butyl-3-methyl imidazolium dimethyl phosphate (IDP), have been studied at the molecular and fibrillar levels. At the molecular level, circular dichroic studies revealed an increase in molar ellipticity values for CS when compared with native collagen, indicating cross-linking, albeit pronounced conformational changes for IDP were witnessed indicating denaturation. The impedance was analyzed to correlate the conformational changes with the hydration dynamics of protein. Changes in the dielectric properties of collagen observed upon treatment with CS and IDP reported molecular reorientation in the surrounding water milieu, suggesting compactness or destabilization of the collagen. This was further confirmed by proton transverse NMR relaxation time measurements, which demonstrated that the water mobility changes in the presence of the ILs. At the fibrillar level, differential scanning calorimetry thermograms for rat tail tendon collagen fibers treated with CS show a 5 °C increase in denaturation temperature, suggesting imparted stability. On the contrary, a significant temperature decrease was noticed for IDP, indicating the destabilization of collagen fibers. The obtained results clearly indicate that the changes in the secondary structure of protein are due to the changes in the hydration dynamics of collagen upon interaction with ILs. Thus, this study on the interaction of collagen with ionic liquids unfolds the propensity of ILs to stabilize or destabilize collagen depending on the changes invoked at the molecular level in terms of structure and dynamics of protein, which also got manifested at the fibrillar level.
中文翻译:

离子液体对胶原蛋白结构和动力学的影响
已经研究了在分子水平和原纤维水平上用两类不同的离子液体硫酸双胆碱(CS)和1-丁基-3-甲基咪唑鎓二甲基磷酸酯(IDP)处理的胶原蛋白的结构和动力学变化。在分子水平上,圆形二向色性研究显示,与天然胶原蛋白相比,CS的摩尔椭圆度值增加,表明发生了交联,尽管观察到IDP的构象发生了明显变化,从而表明了变性。分析了阻抗以使构象变化与蛋白质的水合动力学相关。用CS和IDP处理后观察到的胶原蛋白介电性能的变化报告了周围水环境中的分子重新定向,表明胶原蛋白的致密性或不稳定。质子横向NMR弛豫时间测量进一步证实了这一点,这表明在IL的存在下水的迁移率发生了变化。在原纤维水平,用CS处理的大鼠尾部腱胶原纤维的差示扫描量热法热分析图显示变性温度提高了5°C,表明具有稳定性。相反,IDP的温度显着下降,表明胶原纤维不稳定。所获得的结果清楚地表明,蛋白质二级结构的变化是由于与ILs相互作用时胶原水化动力学变化的结果。因此,
更新日期:2018-01-04
中文翻译:

离子液体对胶原蛋白结构和动力学的影响
已经研究了在分子水平和原纤维水平上用两类不同的离子液体硫酸双胆碱(CS)和1-丁基-3-甲基咪唑鎓二甲基磷酸酯(IDP)处理的胶原蛋白的结构和动力学变化。在分子水平上,圆形二向色性研究显示,与天然胶原蛋白相比,CS的摩尔椭圆度值增加,表明发生了交联,尽管观察到IDP的构象发生了明显变化,从而表明了变性。分析了阻抗以使构象变化与蛋白质的水合动力学相关。用CS和IDP处理后观察到的胶原蛋白介电性能的变化报告了周围水环境中的分子重新定向,表明胶原蛋白的致密性或不稳定。质子横向NMR弛豫时间测量进一步证实了这一点,这表明在IL的存在下水的迁移率发生了变化。在原纤维水平,用CS处理的大鼠尾部腱胶原纤维的差示扫描量热法热分析图显示变性温度提高了5°C,表明具有稳定性。相反,IDP的温度显着下降,表明胶原纤维不稳定。所获得的结果清楚地表明,蛋白质二级结构的变化是由于与ILs相互作用时胶原水化动力学变化的结果。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号