当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessing the Structure of Octastate Molecular Switches Using 1H NMR Density Functional Theory Calculations
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcc.7b11221 Slim Hadj Mohamed 1, 2 , Jean Quertinmont 2 , Stéphanie Delbaere 3 , Lionel Sanguinet 4 , Benoît Champagne 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcc.7b11221 Slim Hadj Mohamed 1, 2 , Jean Quertinmont 2 , Stéphanie Delbaere 3 , Lionel Sanguinet 4 , Benoît Champagne 2
Affiliation
|
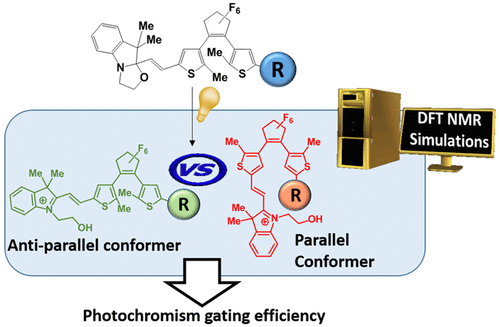
Density functional theory calculations are used to reveal the relationships between the structures, energies, and NMR signatures of an octastate molecular switch composed of a dithienylethene (DTE) unit covalently linked to an indolino[2,1-b]oxazolidine (BOX) moiety through an ethylenic junction. Both the DTE and BOX moieties can adopt open or closed forms. The ethylenic junction can be Z or E, but the latter has been confirmed to be, by far, more stable than the former for all BOX/DTE combinations. In addition, when the DTE is open, the two thienyl units can fold to form parallel conformers, by opposition to the antiparallel or unfolded conformers. Usually parallel conformers present a higher energy than the antiparallel ones, but in the case of compound 2 having a bulky substituent (R = pPh-SMe) on the terminal thienyl group, the enthalpy of one conformer is very close (1–2 kJ mol–1) to that of the most stable antiparallel one, making photocyclization less efficient. These conformational differences and the presence of parallel DTE forms have been substantiated by analyzing experimental 1H NMR chemical shifts in light of their calculated values. These 1H NMR chemical shift calculations led to the following statements: (i) Going from state I (DTE open, BOX closed) to state II (both DTE and BOX are open) the H8 proton of compound 1 (R = Me) is deshielded by ∼0.15 ppm. (ii) The deshielding of H8 proton of compound 2 is larger and attains 0.41 ppm whereas H7 is more shielded by 0.11 ppm. (iii) Then, going from compound 1 to compound 2 leads to deshielding of both H7 and H8 protons. As a consequence, the difference of photochromism gating efficiency among compounds 1, 2, and 3 (R = pPh-OMe) can be attributed to the stabilization of parallel conformer due to an establishment of an intramolecular interaction with BOX opening.
中文翻译:

使用1 H NMR密度泛函理论计算评估八边形分子开关的结构
密度泛函理论计算用于揭示由二噻吩乙烯(DTE)单元共价连接到吲哚并[2,1- b ]恶唑烷(BOX)部分的八噻吩分子开关的结构,能量和NMR特征之间的关系。烯键。DTE和BOX部分都可以采用开放或封闭形式。烯键可以是Z或E,但对于所有BOX / DTE组合,后者都比前者更稳定。另外,当DTE是开放的时,两个噻吩基单元可通过与反平行或未折叠的构象体相反而折叠以形成平行的构象体。通常,平行构象异构体比反平行构象异构体具有更高的能量,但是对于复合构象异构体而言2在末端噻吩基上具有庞大的取代基(R = p Ph-SMe),一个构象异构体的焓与最稳定的反平行异构体的焓非常接近(1-2 kJ mol –1),从而使光环化效率降低。通过根据其计算值分析实验1 H NMR化学位移,可以证实这些构象差异和平行DTE形式的存在。这些1 H NMR化学位移计算得出以下结论:(i)从状态I(DTE打开,BOX闭合)到状态II(DTE和BOX都打开),化合物1的H 8质子(R = Me)被屏蔽约0.15 ppm。(ii)H 8的去屏蔽化合物2的质子更大,达到0.41 ppm,而H 7被0.11 ppm更好地屏蔽。(iii)然后,从化合物1到化合物2导致H 7和H 8质子均脱保护。其结果是,光致变色的选通效率的化合物之间的差1,2,和3(R = p PH-OME)可以归因于构象平行的稳定由于建立了与盒子开口的分子内相互作用的。
更新日期:2018-01-16
中文翻译:

使用1 H NMR密度泛函理论计算评估八边形分子开关的结构
密度泛函理论计算用于揭示由二噻吩乙烯(DTE)单元共价连接到吲哚并[2,1- b ]恶唑烷(BOX)部分的八噻吩分子开关的结构,能量和NMR特征之间的关系。烯键。DTE和BOX部分都可以采用开放或封闭形式。烯键可以是Z或E,但对于所有BOX / DTE组合,后者都比前者更稳定。另外,当DTE是开放的时,两个噻吩基单元可通过与反平行或未折叠的构象体相反而折叠以形成平行的构象体。通常,平行构象异构体比反平行构象异构体具有更高的能量,但是对于复合构象异构体而言2在末端噻吩基上具有庞大的取代基(R = p Ph-SMe),一个构象异构体的焓与最稳定的反平行异构体的焓非常接近(1-2 kJ mol –1),从而使光环化效率降低。通过根据其计算值分析实验1 H NMR化学位移,可以证实这些构象差异和平行DTE形式的存在。这些1 H NMR化学位移计算得出以下结论:(i)从状态I(DTE打开,BOX闭合)到状态II(DTE和BOX都打开),化合物1的H 8质子(R = Me)被屏蔽约0.15 ppm。(ii)H 8的去屏蔽化合物2的质子更大,达到0.41 ppm,而H 7被0.11 ppm更好地屏蔽。(iii)然后,从化合物1到化合物2导致H 7和H 8质子均脱保护。其结果是,光致变色的选通效率的化合物之间的差1,2,和3(R = p PH-OME)可以归因于构象平行的稳定由于建立了与盒子开口的分子内相互作用的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号