当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rearranged Phloroglucinol-Monoterpenoid Adducts from Callistemon rigidus
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00606 Jia-Qing Cao 1 , Hai-Yan Tian 1 , Man-Mei Li 1 , Wei Zhang 1 , Ying Wang 1 , Lei Wang 1 , Wen-Cai Ye 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00606 Jia-Qing Cao 1 , Hai-Yan Tian 1 , Man-Mei Li 1 , Wei Zhang 1 , Ying Wang 1 , Lei Wang 1 , Wen-Cai Ye 1
Affiliation
|
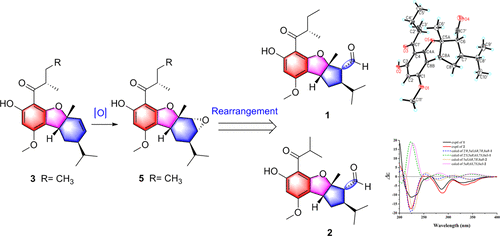
Callisretones A (1) and B (2), two rearranged phloroglucinol-monoterpenoid adducts featuring an unprecedented isopropylcyclopenta[b]benzofuran backbone, together with their postulated biosynthetic precursors (3–9), were isolated from Callistemon rigidus. The previously assigned absolute configurations of viminalins H (7), L (8), and N (9) were revised and unequivocally established by X-ray diffraction data. A putative biosynthetic pathway toward callisretones A and B involving the rearrangement of the terpenoid motif is proposed. In addition, 1 and 2 showed inhibitory effects on nitric oxide production with IC50 values of 15.3 ± 1.0 and 17.7 ± 1.1 μM, respectively.
中文翻译:

重瓣Callistemon的重新排列的间苯三酚-单萜类化合物加合物
Callisretones A(1)和B(2),两个重排的间苯三酚,单萜加合物设有一个前所未有isopropylcyclopenta [ b ]苯并呋喃骨架,与假定的生物合成前体(一起3 - 9)中,从分离千层僵。Viminalins H(7),L(8)和N(9)的先前分配的绝对配置进行了修改,并通过X射线衍射数据明确地确定了这些结构。提出了一个可能的生物合成途径,涉及类萜基序的重排,形成了愈伤组织A和B。此外,1和2表现出对一氧化氮产生的抑制作用,IC 50值分别为15.3±1.0和17.7±1.1μM。
更新日期:2017-12-20
中文翻译:

重瓣Callistemon的重新排列的间苯三酚-单萜类化合物加合物
Callisretones A(1)和B(2),两个重排的间苯三酚,单萜加合物设有一个前所未有isopropylcyclopenta [ b ]苯并呋喃骨架,与假定的生物合成前体(一起3 - 9)中,从分离千层僵。Viminalins H(7),L(8)和N(9)的先前分配的绝对配置进行了修改,并通过X射线衍射数据明确地确定了这些结构。提出了一个可能的生物合成途径,涉及类萜基序的重排,形成了愈伤组织A和B。此外,1和2表现出对一氧化氮产生的抑制作用,IC 50值分别为15.3±1.0和17.7±1.1μM。







































 京公网安备 11010802027423号
京公网安备 11010802027423号