当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-free synthesis of 2,3-dihydrobenzofurans through [4+1] cycloaddition of ortho-hydroxyphenylsubstituted para-quinone methides and sulfur ylides
Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-20 , DOI: 10.1016/j.tet.2017.12.038 Xin-Meng Chen , Ke-Xin Xie , Deng-Feng Yue , Xiao-Mei Zhang , Xiao-Ying Xu , Wei-Cheng Yuan
中文翻译:

通过[4 + 1]环加成的2,3-二氢苯并呋喃催化剂-自由合成邻-hydroxyphenylsubstituted对-醌的甲基化物和硫叶立德
更新日期:2017-12-20
Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-20 , DOI: 10.1016/j.tet.2017.12.038 Xin-Meng Chen , Ke-Xin Xie , Deng-Feng Yue , Xiao-Mei Zhang , Xiao-Ying Xu , Wei-Cheng Yuan
|
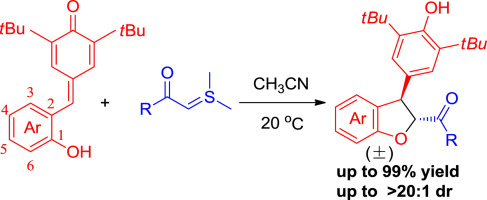
An efficient [4+1] cycloaddition of ortho-hydroxyphenylsubstituted para-quinone methides and sulfur ylides was achieved under the catalyst-free condition. With this developed protocol, a series of trans-2,3-dihydrobenzofurans were obtained in excellent yields (up to 99%) with high diastereoselectivities (>20:1 dr). The usefulness of the protocol was also demonstrated by the versatile conversions of the 2,3-dihydrobenzofurans into other functionalized benzofurans.
中文翻译:

通过[4 + 1]环加成的2,3-二氢苯并呋喃催化剂-自由合成邻-hydroxyphenylsubstituted对-醌的甲基化物和硫叶立德
的有效[4 + 1]环加成邻-hydroxyphenylsubstituted对-醌的甲基化物和硫叶立德是在无催化剂的条件下实现的。通过这种发达的方案,可以以高收率(> 20:1 dr)以优异的收率(高达99%)获得一系列反式-2,3-二氢苯并呋喃。2,3-二氢苯并呋喃向其他官能化苯并呋喃的通用转化也证明了该方案的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号