当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adiabatic and Nonadiabatic Charge Transport in Li–S Batteries
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.chemmater.7b04618 Haesun Park , Nitin Kumar , Marko Melander 1, 2 , Tejs Vegge 1 , Juan Maria Garcia Lastra 1 , Donald J. Siegel 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.chemmater.7b04618 Haesun Park , Nitin Kumar , Marko Melander 1, 2 , Tejs Vegge 1 , Juan Maria Garcia Lastra 1 , Donald J. Siegel 1
Affiliation
|
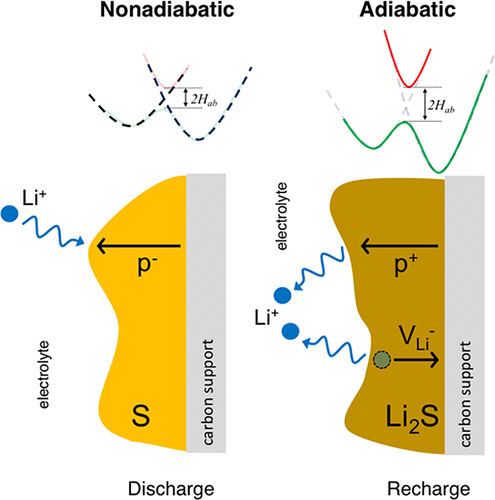
The insulating nature of the redox end members in Li–S batteries, α-S and Li2S, has the potential to limit the capacity and efficiency of this emerging energy storage system. Nevertheless, the mechanisms responsible for ionic and electronic transport in these materials remain a matter of debate. The present study clarifies these mechanisms—in both the adiabatic and nonadiabatic charge transfer regimes—by employing a combination of hybrid-functional-based and constrained density functional theory calculations. Charge transfer in Li2S is predicted to be adiabatic, and thus is well-described by conventional DFT methodologies. In sulfur, however, transitions between S8 rings are nonadiabatic. In this case, conventional DFT overestimates charge transfer rates by up to 2 orders of magnitude. Delocalized holes, and to a lesser extent, localized electron polarons, are predicted to be the most mobile electronic charge carriers in α-S; in Li2S, hole polarons dominate. Although all carriers exhibit extremely low equilibrium concentrations, and thus yield negligible contributions to the conductivity, their mobilities are sufficient to enable the sulfur loading targets necessary for high energy densities. Our results highlight the value of methods capable of capturing nonadiabaticity, such as constrained DFT. These techniques are especially important for molecular crystals such as α-S, where longer-range charge transfer events are expected. Combining the present computational results with prior experimental studies, we conclude that low equilibrium carrier concentrations are responsible for sluggish charge transport in α-S and Li2S. Thus, a potential strategy for improving the performance of Li–S batteries is to increase the concentrations of holes in these redox end members.
中文翻译:

锂电池的绝热和非绝热电荷传输
Li-S电池(α-S和Li 2 S)中氧化还原末端成员的绝缘性质可能会限制这种新兴储能系统的容量和效率。然而,负责这些材料中离子和电子传输的机制仍存在争议。本研究通过结合基于混合功能和受限密度泛函理论计算的方法,在绝热和非绝热电荷转移机制中阐明了这些机理。预测Li 2 S中的电荷转移是绝热的,因此可以通过常规DFT方法很好地描述。然而,在硫中,S 8之间的过渡环是非绝热的。在这种情况下,传统的DFT高估了电荷传输速率多达2个数量级。据预测,离域空穴和(在较小程度上)局部电子极化子是α-S中最易移动的电子电荷载流子。在李2S,孔极化子占主导地位。尽管所有载流子均表现出极低的平衡浓度,因此对电导率的贡献可忽略不计,但它们的迁移率足以实现高能量密度所需的硫载量目标。我们的结果突出了能够捕获非绝热性的方法(例如约束DFT)的价值。这些技术对于诸如α-S之类的分子晶体特别重要,因为在这种晶体中预期会有更长的电荷转移事件。结合当前的计算结果和先前的实验研究,我们得出结论,低平衡载流子浓度是造成α-S和Li 2中电荷迁移缓慢的原因S.因此,改善Li-S电池性能的一种潜在策略是增加这些氧化还原末端构件中孔的浓度。
更新日期:2018-01-16
中文翻译:

锂电池的绝热和非绝热电荷传输
Li-S电池(α-S和Li 2 S)中氧化还原末端成员的绝缘性质可能会限制这种新兴储能系统的容量和效率。然而,负责这些材料中离子和电子传输的机制仍存在争议。本研究通过结合基于混合功能和受限密度泛函理论计算的方法,在绝热和非绝热电荷转移机制中阐明了这些机理。预测Li 2 S中的电荷转移是绝热的,因此可以通过常规DFT方法很好地描述。然而,在硫中,S 8之间的过渡环是非绝热的。在这种情况下,传统的DFT高估了电荷传输速率多达2个数量级。据预测,离域空穴和(在较小程度上)局部电子极化子是α-S中最易移动的电子电荷载流子。在李2S,孔极化子占主导地位。尽管所有载流子均表现出极低的平衡浓度,因此对电导率的贡献可忽略不计,但它们的迁移率足以实现高能量密度所需的硫载量目标。我们的结果突出了能够捕获非绝热性的方法(例如约束DFT)的价值。这些技术对于诸如α-S之类的分子晶体特别重要,因为在这种晶体中预期会有更长的电荷转移事件。结合当前的计算结果和先前的实验研究,我们得出结论,低平衡载流子浓度是造成α-S和Li 2中电荷迁移缓慢的原因S.因此,改善Li-S电池性能的一种潜在策略是增加这些氧化还原末端构件中孔的浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号