当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Suzuki–Miyaura Coupling of Aliphatic Amides
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acscatal.7b03688 Timothy B Boit 1 , Nicholas A Weires 1 , Junyong Kim 1 , Neil K Garg 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acscatal.7b03688 Timothy B Boit 1 , Nicholas A Weires 1 , Junyong Kim 1 , Neil K Garg 1
Affiliation
|
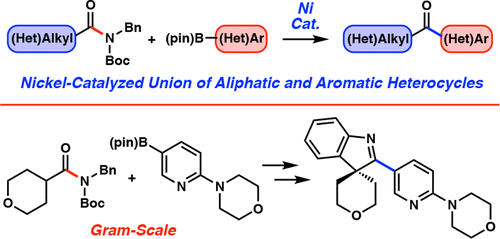
We report the Ni-catalyzed Suzuki–Miyaura coupling of aliphatic amide derivatives. Prior studies have shown that aliphatic amide derivatives can undergo Ni-catalyzed carbon–heteroatom bond formation but that Ni-mediated C–C bond formation using aliphatic amide derivatives has remained difficult. The coupling disclosed herein is tolerant of considerable variation with respect to both the amide-based substrate and the boronate coupling partner and proceeds in the presence of heterocycles and epimerizable stereocenters. Moreover, a gram-scale Suzuki–Miyaura coupling/Fischer indolization sequence demonstrates the ease with which unique polyheterocyclic scaffolds can be constructed, particularly by taking advantage of the enolizable ketone functionality present in the cross-coupled product. The methodology provides an efficient means to form C–C bonds from aliphatic amide derivatives using nonprecious-metal catalysis and offers a general platform for the heteroarylation of aliphatic acyl electrophiles.
中文翻译:

镍催化脂肪族酰胺的 Suzuki-Miyaura 偶联
我们报道了镍催化的脂肪族酰胺衍生物的 Suzuki-Miyaura 偶联。先前的研究表明,脂肪族酰胺衍生物可以进行镍催化的碳-杂原子键形成,但使用脂肪族酰胺衍生物形成镍介导的C-C键仍然很困难。本文公开的偶联对于基于酰胺的底物和硼酸酯偶联配偶体而言能够耐受相当大的变化,并且在杂环和可差向异构的立构中心存在下进行。此外,克级的 Suzuki-Miyaura 偶联/Fischer 吲哚化序列表明可以轻松构建独特的多杂环支架,特别是通过利用交叉偶联产物中存在的烯醇化酮官能团。该方法提供了一种使用非贵金属催化从脂肪族酰胺衍生物形成C-C键的有效方法,并为脂肪族酰基亲电子试剂的杂芳基化提供了通用平台。
更新日期:2018-01-05
中文翻译:

镍催化脂肪族酰胺的 Suzuki-Miyaura 偶联
我们报道了镍催化的脂肪族酰胺衍生物的 Suzuki-Miyaura 偶联。先前的研究表明,脂肪族酰胺衍生物可以进行镍催化的碳-杂原子键形成,但使用脂肪族酰胺衍生物形成镍介导的C-C键仍然很困难。本文公开的偶联对于基于酰胺的底物和硼酸酯偶联配偶体而言能够耐受相当大的变化,并且在杂环和可差向异构的立构中心存在下进行。此外,克级的 Suzuki-Miyaura 偶联/Fischer 吲哚化序列表明可以轻松构建独特的多杂环支架,特别是通过利用交叉偶联产物中存在的烯醇化酮官能团。该方法提供了一种使用非贵金属催化从脂肪族酰胺衍生物形成C-C键的有效方法,并为脂肪族酰基亲电子试剂的杂芳基化提供了通用平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号