当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study of Small Iron–Oxyhydroxide Clusters and Formation of Ferrihydrite
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jpca.7b09470 Bidisa Das 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jpca.7b09470 Bidisa Das 1
Affiliation
|
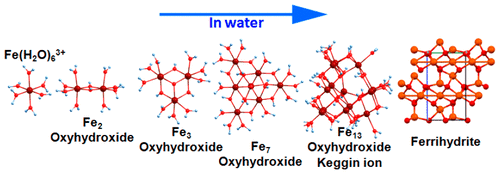
Hydrolysis of iron compounds in water leads to the formation of Fe(III) oyxhydroxide-based minerals like ferrihydrite, which act as natural scavengers of inorganic contaminants in the environment. Though studied widely, experimental identification of these oxyhydroxides remains very difficult due to their extreme reactivity. The present study theoretically investigates the formation of Fe(III) oxyhydroxides starting from a single hydrated Fe(III) ion, modeling the formation of larger clusters gradually. The structures, formation enthalpies, and free energies of dimers, trimers, tetramers, and even larger Fe(III) oxyhydroxide clusters comprising of Fe5, Fe7, and Fe13–Keggin ions in gaseous phase and in aqueous medium (using self-consistent reaction field method) are systematically studied using density functional theory. Spontaneous formation of certain multinuclear Fe(III) oxyhydroxide clusters with clear structural signatures of ferrihydrite highlights their potential as prenucleation clusters in the course of mineralization.
中文翻译:

铁羟基氧化物小团簇和水铁矿形成的理论研究
铁化合物在水中的水解导致形成基于Fe(III)羟基氧化铁的矿物(如亚铁酸盐),这些矿物可作为环境中无机污染物的天然清除剂。尽管已进行了广泛的研究,但由于它们的极高反应活性,对这些羟基氧化物的实验鉴定仍然非常困难。本研究从理论上研究了从单个水合Fe(III)离子开始形成羟基氢氧化Fe(III)的过程,逐步模拟了较大簇的形成。二聚体,三聚体,四聚体,甚至更大的由Fe 5,Fe 7和Fe 13组成的羟基氢氧化铁(III)的结构,形成焓和自由能–使用密度泛函理论系统地研究了气相和水介质中的乞金离子(使用自洽反应场方法)。自发形成某些具有清晰水铁矿结构特征的多核氢氧化三价铁(III)簇,突显了它们在成矿过程中作为预成核簇的潜力。
更新日期:2018-01-08
中文翻译:

铁羟基氧化物小团簇和水铁矿形成的理论研究
铁化合物在水中的水解导致形成基于Fe(III)羟基氧化铁的矿物(如亚铁酸盐),这些矿物可作为环境中无机污染物的天然清除剂。尽管已进行了广泛的研究,但由于它们的极高反应活性,对这些羟基氧化物的实验鉴定仍然非常困难。本研究从理论上研究了从单个水合Fe(III)离子开始形成羟基氢氧化Fe(III)的过程,逐步模拟了较大簇的形成。二聚体,三聚体,四聚体,甚至更大的由Fe 5,Fe 7和Fe 13组成的羟基氢氧化铁(III)的结构,形成焓和自由能–使用密度泛函理论系统地研究了气相和水介质中的乞金离子(使用自洽反应场方法)。自发形成某些具有清晰水铁矿结构特征的多核氢氧化三价铁(III)簇,突显了它们在成矿过程中作为预成核簇的潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号