当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Chiral α-Thio-Quaternary Stereogenic Centers via Phase-Transfer-Catalyzed α-Alkylation of α-Acylthiomalonates
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02605 Min Woo Ha 1 , Jun Young Lee 1 , Doyoung Kim 1 , Geumwoo Lee 1 , Jae Kyun Lee 2 , Suckchang Hong 1 , Hyeung-geun Park 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02605 Min Woo Ha 1 , Jun Young Lee 1 , Doyoung Kim 1 , Geumwoo Lee 1 , Jae Kyun Lee 2 , Suckchang Hong 1 , Hyeung-geun Park 1
Affiliation
|
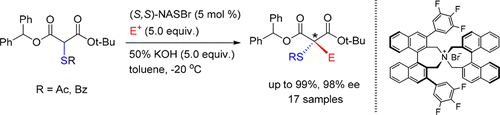
An efficient synthetic method for establishing chiral α-thio-α-quaternary stereogenic center was successfully developed. The enantioselective α-alkylation of α-acylthiomalonates under phase-transfer catalytic conditions [50% aq. KOH, toluene, −20 °C, and (S,S)-3,4,5-trifluorophenyl-NAS bromide] provided the corresponding α-acylthio-α-alkylmalonates in high chemical yields (up to 99%) and high optical yields (up to 98% ee).
中文翻译:

通过相转移催化的α-酰基硫代丙二酸酯的α-烷基化手性α-硫-季位立体中心的对映选择性合成
成功开发了一种建立手性α-硫代-α-四元立体异构中心的有效合成方法。α-酰基硫代丙二酸酯在相转移催化条件下[50%aq。KOH,甲苯,-20°C和(S,S)-3,4,5-三氟苯基-NAS溴化物]以高化学产率(高达99%)和高光学产率提供了相应的α-酰基硫代-α-烷基丙二酸酯产量(高达98%ee)。
更新日期:2018-01-10
中文翻译:

通过相转移催化的α-酰基硫代丙二酸酯的α-烷基化手性α-硫-季位立体中心的对映选择性合成
成功开发了一种建立手性α-硫代-α-四元立体异构中心的有效合成方法。α-酰基硫代丙二酸酯在相转移催化条件下[50%aq。KOH,甲苯,-20°C和(S,S)-3,4,5-三氟苯基-NAS溴化物]以高化学产率(高达99%)和高光学产率提供了相应的α-酰基硫代-α-烷基丙二酸酯产量(高达98%ee)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号