当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into structure and dynamics of (Mn,Fe)Ox-promoted Rh nanoparticles
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-12-20 , DOI: 10.1039/c7fd00215g Maria Dimitrakopoulou 1, 2, 3 , Xing Huang 1, 2, 3 , Jutta Kröhnert 1, 2, 3 , Detre Teschner 1, 2, 3, 4, 5 , Sebastian Praetz 3, 6, 7, 8 , Christopher Schlesiger 3, 6, 7, 8 , Wolfgang Malzer 3, 6, 7, 8 , Christiane Janke 3, 9, 10, 11, 12 , Ekkehard Schwab 3, 9, 10, 11, 12 , Frank Rosowski 3, 9, 10, 11, 12 , Harry Kaiser 3, 13, 14 , Stephan Schunk 3, 13, 14 , Robert Schlögl 1, 2, 3, 4, 5 , Annette Trunschke 1, 2, 3
Faraday Discussions ( IF 3.3 ) Pub Date : 2017-12-20 , DOI: 10.1039/c7fd00215g Maria Dimitrakopoulou 1, 2, 3 , Xing Huang 1, 2, 3 , Jutta Kröhnert 1, 2, 3 , Detre Teschner 1, 2, 3, 4, 5 , Sebastian Praetz 3, 6, 7, 8 , Christopher Schlesiger 3, 6, 7, 8 , Wolfgang Malzer 3, 6, 7, 8 , Christiane Janke 3, 9, 10, 11, 12 , Ekkehard Schwab 3, 9, 10, 11, 12 , Frank Rosowski 3, 9, 10, 11, 12 , Harry Kaiser 3, 13, 14 , Stephan Schunk 3, 13, 14 , Robert Schlögl 1, 2, 3, 4, 5 , Annette Trunschke 1, 2, 3
Affiliation
|
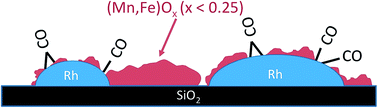
The mutual interaction between Rh nanoparticles and manganese/iron oxide promoters in silica-supported Rh catalysts for the hydrogenation of CO to higher alcohols was analyzed by applying a combination of integral techniques including temperature-programmed reduction (TPR), X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS) and Fourier transform infrared (FTIR) spectroscopy with local analysis by using high angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) in combination with energy dispersive X-ray spectroscopy (EDX). The promoted catalysts show reduced CO adsorption capacity as evidenced through FTIR spectroscopy, which is attributed to a perforated core–shell structure of the Rh nano-particles in accordance with the microstructural analysis from electron microscopy. Iron and manganese occur in low formal oxidation states between 2+ and zero in the reduced catalysts as shown by using TPR and XAS. Infrared spectroscopy measured in diffuse reflectance at reaction temperature and pressure indicates that partial coverage of the Rh particles is maintained at reaction temperature under operation and that the remaining accessible metal adsorption sites might be catalytically less relevant because the hydrogenation of adsorbed carbonyl species at 523 K and 30 bar hydrogen essentially failed. It is concluded that Rh0 is poisoned due to the adsorption of CO under the reaction conditions of CO hydrogenation. The active sites are associated either with a (Mn,Fe)Ox (x < 0.25) phase or species at the interface between Rh and its co-catalyst (Mn,Fe)Ox.
中文翻译:

对(Mn,Fe)O x促进的Rh纳米颗粒的结构和动力学的见解
通过应用包括程序升温还原(TPR),X射线光电子能谱( XPS),X射线吸收光谱(XAS)和傅里叶变换红外(FTIR)光谱结合使用高角度环形暗场扫描透射电子显微镜(HAADF-STEM)和能量色散X射线光谱(EDX)进行局部分析)。FTIR光谱表明,促进的催化剂显示出降低的CO吸附能力,这归因于根据电子显微镜的微观结构分析,Rh纳米颗粒的穿孔的核-壳结构。如使用TPR和XAS所示,在还原的催化剂中,铁和锰以2+到0的低形式氧化态出现。在反应温度和压力下以漫反射率测量的红外光谱表明,在操作条件下,Rh颗粒的部分覆盖保持在反应温度下,并且剩余的可及金属吸附位点在催化上可能不太相关,因为在523 K和30 bar氢气基本失效。结论是Rh 在反应温度和压力下以漫反射率测量的红外光谱表明,在操作条件下,Rh颗粒的部分覆盖保持在反应温度下,并且剩余的可及金属吸附位点在催化上可能不太相关,因为在523 K和30 bar氢气基本失效。结论是Rh 在反应温度和压力下以漫反射率测量的红外光谱表明,在操作条件下,Rh颗粒的部分覆盖保持在反应温度下,并且剩余的可及金属吸附位点在催化上可能不太相关,因为在523 K和30 bar氢气基本失效。结论是Rh在CO加氢反应条件下,由于CO的吸附,使0中毒。活性位点与(Mn,Fe)O x( x <0.25)相或在Rh及其助催化剂(Mn,Fe)O x之间的界面处的物种相关。
更新日期:2018-09-03
中文翻译:

对(Mn,Fe)O x促进的Rh纳米颗粒的结构和动力学的见解
通过应用包括程序升温还原(TPR),X射线光电子能谱( XPS),X射线吸收光谱(XAS)和傅里叶变换红外(FTIR)光谱结合使用高角度环形暗场扫描透射电子显微镜(HAADF-STEM)和能量色散X射线光谱(EDX)进行局部分析)。FTIR光谱表明,促进的催化剂显示出降低的CO吸附能力,这归因于根据电子显微镜的微观结构分析,Rh纳米颗粒的穿孔的核-壳结构。如使用TPR和XAS所示,在还原的催化剂中,铁和锰以2+到0的低形式氧化态出现。在反应温度和压力下以漫反射率测量的红外光谱表明,在操作条件下,Rh颗粒的部分覆盖保持在反应温度下,并且剩余的可及金属吸附位点在催化上可能不太相关,因为在523 K和30 bar氢气基本失效。结论是Rh 在反应温度和压力下以漫反射率测量的红外光谱表明,在操作条件下,Rh颗粒的部分覆盖保持在反应温度下,并且剩余的可及金属吸附位点在催化上可能不太相关,因为在523 K和30 bar氢气基本失效。结论是Rh 在反应温度和压力下以漫反射率测量的红外光谱表明,在操作条件下,Rh颗粒的部分覆盖保持在反应温度下,并且剩余的可及金属吸附位点在催化上可能不太相关,因为在523 K和30 bar氢气基本失效。结论是Rh在CO加氢反应条件下,由于CO的吸附,使0中毒。活性位点与(Mn,Fe)O x( x <0.25)相或在Rh及其助催化剂(Mn,Fe)O x之间的界面处的物种相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号