Biomaterials ( IF 12.8 ) Pub Date : 2017-12-20 , DOI: 10.1016/j.biomaterials.2017.12.015 Xiao Zhao , Xiuchao Wang , Wei Sun , Keman Cheng , Hao Qin , Xuexiang Han , Yu Lin , Yongwei Wang , Jiayan Lang , Ruifang Zhao , Xiaowei Zheng , Ying Zhao , Jian shi , Jihui Hao , Qing Robert Miao , Guangjun Nie , He Ren
|
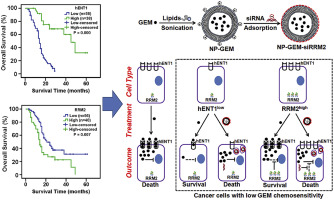
Low chemosensitivity considerably restricts the therapeutic efficacy of gemcitabine (GEM) in pancreatic cancer treatment. Using immunohistochemical evaluation, we investigated that decreased expression of human equilibrative nucleoside transporter-1 (hENT1, which is the major GEM transporter across cell membranes) and increased expression of ribonucleotide reductase subunit 2 (RRM2, which decreases the cytotoxicity of GEM) was associated with low GEM chemosensitivity. To solve these problems, we employed a nanomedicine-based formulation of cationic liposomes for co-delivery of GEM along with siRNA targeting RRM2. Due to the specific endocytic uptake mechanism of nanocarriers and gene-silencing effect of RRM2 siRNA, this nanomedicine formulation significantly increased GEM chemosensitivity in tumor models of genetically engineered Panc1 cells with low hENT1 or high RRM2 expression. Moreover, in a series of patient-derived cancer cells, we demonstrated that the therapeutic benefits of the nanomedicine formulations were associated with the expression levels of hENT1 and RRM2. In summary, we found that the essential factors of GEM chemosensitivity were the expression levels of hENT1 and RRM2, and synthesized nanoformulations can overcome these problems. This unique design of nanomedicine not only provides a universal platform to enhance chemosensitivity but also contributes to the precision design and personalized treatment in nanomedicine.
中文翻译:

纳米药物的精确设计可恢复吉西他滨的化学敏感性,用于个性化胰腺导管腺癌治疗。
低化学敏感性极大地限制了吉西他滨(GEM)在胰腺癌治疗中的治疗效果。使用免疫组化评估,我们调查了人类平衡核苷转运蛋白-1(hENT1,这是跨细胞膜的主要GEM转运蛋白)的表达降低和核糖核苷酸还原酶亚基2(RRM2,其降低了GEM的细胞毒性)的表达增加与GEM化学敏感性低。为了解决这些问题,我们采用了基于纳米药物的阳离子脂质体制剂,与靶向RRM2的siRNA一起共同递送GEM。由于纳米载体的特定内吞摄取机制和RRM2 siRNA的基因沉默效应,在具有低hENT1或高RRM2表达的基因改造Panc1细胞的肿瘤模型中,这种纳米药物制剂显着提高了GEM的化学敏感性。此外,在一系列患者来源的癌细胞中,我们证明了纳米药物制剂的治疗优势与hENT1和RRM2的表达水平有关。总而言之,我们发现GEM化学敏感性的主要因素是hENT1和RRM2的表达水平,合成的纳米制剂可以克服这些问题。纳米药物的这种独特设计不仅提供了增强化学敏感性的通用平台,而且还为纳米药物的精确设计和个性化治疗做出了贡献。我们证明了纳米药物制剂的治疗益处与hENT1和RRM2的表达水平有关。总而言之,我们发现GEM化学敏感性的主要因素是hENT1和RRM2的表达水平,合成的纳米制剂可以克服这些问题。纳米药物的这种独特设计不仅提供了增强化学敏感性的通用平台,而且还为纳米药物的精确设计和个性化治疗做出了贡献。我们证明了纳米药物制剂的治疗益处与hENT1和RRM2的表达水平有关。总而言之,我们发现GEM化学敏感性的主要因素是hENT1和RRM2的表达水平,合成的纳米制剂可以克服这些问题。纳米药物的这种独特设计不仅提供了增强化学敏感性的通用平台,而且还为纳米药物的精确设计和个性化治疗做出了贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号