当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of carba-cyclophellitols: a new class of carbohydrate mimetics
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-02-14 , DOI: 10.1002/ejoc.201701601 Thomas J. M. Beenakker 1 , Dennis P. A. Wander 1 , Jeroen D. C. Codée 1 , Johannes M. F. G. Aerts 2 , Gijsbert A. van der Marel 1 , Herman S. Overkleeft 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2018-02-14 , DOI: 10.1002/ejoc.201701601 Thomas J. M. Beenakker 1 , Dennis P. A. Wander 1 , Jeroen D. C. Codée 1 , Johannes M. F. G. Aerts 2 , Gijsbert A. van der Marel 1 , Herman S. Overkleeft 1
Affiliation
|
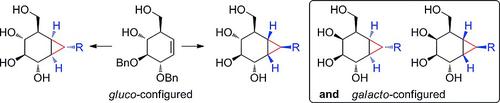
Cyclophellitol and cyclophellitol aziridine are potent and irreversible inhibitors of retaining β-glucosidases. They preferentially adopt a 4H3 half-chair conformation, thereby mimicking the substrate-transition-state conformation characteristic of retaining β-glucosidases. As a consequence, both compounds bind tightly to the enzyme active site, and attack of the catalytic nucleophile onto the epoxide/aziridine results in enzyme deactivation. Replacement of the epoxide oxygen in cyclophellitol by a (substituted) carbon yielded carba-cyclophellitols, a conceptually new class of inhibitors of retaining β-glucosidases, as we demonstrated in a recent communication. In this paper, in-depth synthetic studies of this class of compounds are described, and the preparation of a comprehensive set of structurally and configurationally new carba-cyclophellitols is presented.
中文翻译:

carba-cyclophellitols 的合成:一类新的碳水化合物模拟物
Cyclophellitol 和 cyclophellitol aziridine 是保留 β-葡萄糖苷酶的有效且不可逆的抑制剂。它们优先采用 4H3 半椅构象,从而模仿保留 β-葡萄糖苷酶的底物-过渡态构象特征。因此,两种化合物都与酶活性位点紧密结合,催化亲核试剂对环氧化物/氮丙啶的攻击导致酶失活。正如我们在最近的通讯中所证明的那样,用(取代的)碳取代环酚醇中的环氧化物氧产生了卡巴环酚醇,这是一种概念上新的保留 β-葡糖苷酶的抑制剂。在本文中,描述了对此类化合物的深入合成研究,
更新日期:2018-02-14
中文翻译:

carba-cyclophellitols 的合成:一类新的碳水化合物模拟物
Cyclophellitol 和 cyclophellitol aziridine 是保留 β-葡萄糖苷酶的有效且不可逆的抑制剂。它们优先采用 4H3 半椅构象,从而模仿保留 β-葡萄糖苷酶的底物-过渡态构象特征。因此,两种化合物都与酶活性位点紧密结合,催化亲核试剂对环氧化物/氮丙啶的攻击导致酶失活。正如我们在最近的通讯中所证明的那样,用(取代的)碳取代环酚醇中的环氧化物氧产生了卡巴环酚醇,这是一种概念上新的保留 β-葡糖苷酶的抑制剂。在本文中,描述了对此类化合物的深入合成研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号