当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α‐Aminoxy‐Acid‐Auxiliary‐Enabled Intermolecular Radical γ‐C(sp3)−H Functionalization of Ketones
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-15 , DOI: 10.1002/anie.201712066 Heng Jiang 1 , Armido Studer 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-15 , DOI: 10.1002/anie.201712066 Heng Jiang 1 , Armido Studer 1
Affiliation
|
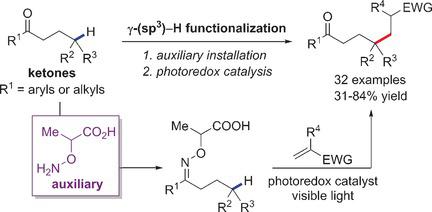
A method for site‐specific intermolecular γ‐C(sp3)−H functionalization of ketones has been developed using an α‐aminoxy acid auxiliary applying photoredox catalysis. Regioselective activation of an inert C−H bond is achieved by 1,5‐hydrogen atom abstraction by an oxidatively generated iminyl radical. Tertiary and secondary C‐radicals thus formed at the γ‐position of the imine functionality undergo radical conjugate addition to various Michael acceptors to provide, after reduction and imine hydrolysis, the corresponding γ‐functionalized ketones.
中文翻译:

酮的α-氨氧基-酸-辅助分子间自由基γ-C(sp3)-H官能化
已开发出一种使用光还原氧化催化的α-氨基羟酸助剂对酮进行位点特异性分子间γ-C(sp 3)-H官能化的方法。惰性CH键的区域选择性激活是通过氧化生成的亚氨基自由基提取1,5-氢原子来实现的。在亚胺官能团的γ位置上形成的叔和仲C自由基经过自由基共轭加成到各种Michael受体上,以在还原和亚胺水解后提供相应的γ官能化的酮。
更新日期:2018-01-15
中文翻译:

酮的α-氨氧基-酸-辅助分子间自由基γ-C(sp3)-H官能化
已开发出一种使用光还原氧化催化的α-氨基羟酸助剂对酮进行位点特异性分子间γ-C(sp 3)-H官能化的方法。惰性CH键的区域选择性激活是通过氧化生成的亚氨基自由基提取1,5-氢原子来实现的。在亚胺官能团的γ位置上形成的叔和仲C自由基经过自由基共轭加成到各种Michael受体上,以在还原和亚胺水解后提供相应的γ官能化的酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号