当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ electrochromic efficiency of a nickel oxide thin film: origin of electrochemical process and electrochromic degradation†
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1039/c7tc04696k Qirong Liu 1, 2, 3, 4, 5 , Qianqian Chen 3, 4, 5, 6, 7 , Qianqian Zhang 1, 2, 3, 4, 5 , Yu Xiao 1, 2, 3, 4, 5 , Xiaolan Zhong 1, 2, 3, 4, 5 , Guobo Dong 1, 2, 3, 4, 5 , Marie-Paule Delplancke-Ogletree 3, 4, 5, 8, 9 , Herman Terryn 10, 11, 12, 13 , Kitty Baert 10, 11, 12, 13 , François Reniers 3, 4, 5, 6, 7 , Xungang Diao 1, 2, 3, 4, 5
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1039/c7tc04696k Qirong Liu 1, 2, 3, 4, 5 , Qianqian Chen 3, 4, 5, 6, 7 , Qianqian Zhang 1, 2, 3, 4, 5 , Yu Xiao 1, 2, 3, 4, 5 , Xiaolan Zhong 1, 2, 3, 4, 5 , Guobo Dong 1, 2, 3, 4, 5 , Marie-Paule Delplancke-Ogletree 3, 4, 5, 8, 9 , Herman Terryn 10, 11, 12, 13 , Kitty Baert 10, 11, 12, 13 , François Reniers 3, 4, 5, 6, 7 , Xungang Diao 1, 2, 3, 4, 5
Affiliation
|
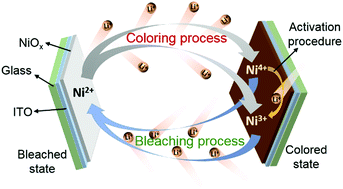
Electrochromic nickel oxide (NiOx) thin films are one of the most promising anodic colored materials. However, there is lack of accurate description of their electrochemical process and degradation mechanism. In this study, a novel approach involving in situ electrochromic efficiency is proposed to reveal the electrochemical origin of an electrochromic NiOx thin film cycled in a Li+-ion electrolyte. The results indicate that the coloring process of the NiOx thin film refers to the oxidation reactions of Ni2+ to Ni3+ and Ni2+ to Ni4+ (in two forms of Ni3O4 and Ni2O3), and the bleaching process is associated with the reduction reactions of Ni4+ to Ni2+, Ni4+ to Ni3+, and Ni3+ to Ni2+. The irreversible reduction of Ni4+ to Ni3+ plays a dominant role in the activation procedure of NiOx. It is deduced that the Li+-ion trapping in the bleaching process along with the reduction reactions of Ni4+ to Ni3+ and Ni3+ to Ni2+ causes the degradation of the electrochromic properties. This study provides a further insight into the electrochromic mechanism and is conducive to the improvement of the long-term cyclic durability for Li+-based electrochromic NiOx. Moreover, the study significantly establishes a direct connection between an electrochemical process and a variation in the optical absorbance of materials.
中文翻译:

氧化镍薄膜的原位电致变色效率:电化学过程和电致变色降解的起源†
电致变色氧化镍(NiO x)薄膜是最有前途的阳极有色材料之一。但是,缺乏对其电化学过程和降解机理的准确描述。在这项研究中,提出了一种涉及原位电致变色效率的新方法,以揭示在Li +离子电解质中循环的电致变色NiO x薄膜的电化学起源。结果表明,NiO x薄膜的着色过程是指Ni 2+转化为Ni 3+和Ni 2+转化为Ni 4+(两种形式的Ni 3 O 4)的氧化反应。和Ni 2 ö 3),和漂白过程与镍的还原反应相关4+与Ni 2+,镍4+对于Ni 3+和Ni 3+与Ni 2+。Ni 4+不可还原地还原为Ni 3+在NiO x的活化过程中起主要作用。推论出漂白过程中Li +离子的俘获以及Ni 4+还原为Ni 3+和Ni 3+还原为Ni 2+的反应。引起电致变色性能的下降。这项研究为电致变色机理提供了进一步的见解,并有助于提高基于Li +的电致变色NiO x的长期循环耐久性。而且,该研究显着地建立了电化学过程与材料的光吸收率变化之间的直接联系。
更新日期:2017-12-20
中文翻译:

氧化镍薄膜的原位电致变色效率:电化学过程和电致变色降解的起源†
电致变色氧化镍(NiO x)薄膜是最有前途的阳极有色材料之一。但是,缺乏对其电化学过程和降解机理的准确描述。在这项研究中,提出了一种涉及原位电致变色效率的新方法,以揭示在Li +离子电解质中循环的电致变色NiO x薄膜的电化学起源。结果表明,NiO x薄膜的着色过程是指Ni 2+转化为Ni 3+和Ni 2+转化为Ni 4+(两种形式的Ni 3 O 4)的氧化反应。和Ni 2 ö 3),和漂白过程与镍的还原反应相关4+与Ni 2+,镍4+对于Ni 3+和Ni 3+与Ni 2+。Ni 4+不可还原地还原为Ni 3+在NiO x的活化过程中起主要作用。推论出漂白过程中Li +离子的俘获以及Ni 4+还原为Ni 3+和Ni 3+还原为Ni 2+的反应。引起电致变色性能的下降。这项研究为电致变色机理提供了进一步的见解,并有助于提高基于Li +的电致变色NiO x的长期循环耐久性。而且,该研究显着地建立了电化学过程与材料的光吸收率变化之间的直接联系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号