JAMA Surgery ( IF 15.7 ) Pub Date : 2018-05-01 , DOI: 10.1001/jamasurg.2017.5040 Vatche G. Agopian 1 , Michael P. Harlander-Locke 1 , Daniela Markovic 2 , Wethit Dumronggittigule 1, 3 , Victor Xia 4 , Fady M. Kaldas 1 , Ali Zarrinpar 1 , Hasan Yersiz 1 , Douglas G. Farmer 1 , Jonathan R. Hiatt 1 , Ronald W. Busuttil 1
|
Importance Early allograft dysfunction (EAD) following a liver transplant (LT) unequivocally portends adverse graft and patient outcomes, but a widely accepted classification or grading system is lacking.
Objective To develop a model for individualized risk estimation of graft failure after LT and then compare the model’s prognostic performance with the existing binary EAD definition (bilirubin level of ≥10 mg/dL on postoperative day 7, international normalized ratio of ≥1.6 on postoperative day 7, or aspartate aminotransferase or alanine aminotransferase level of >2000 U/L within the first 7 days) and the Model for Early Allograft Function (MEAF) score.
Design, Setting, and Participants This retrospective single-center analysis used a transplant database to identify all adult patients who underwent a primary LT and had data on 10 days of post-LT laboratory variables at the Dumont-UCLA Transplant Center of the David Geffen School of Medicine at UCLA between February 1, 2002, and June 30, 2015. Data collection took place from January 4, 2016, to June 30, 2016. Data analysis was conducted from July 1, 2016, to August 30, 2017.
Main Outcomes and Measures Three-month graft failure–free survival.
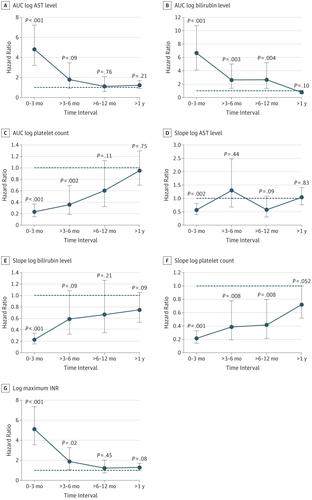
Results Of 2021 patients who underwent primary LT over the study period, 2008 (99.4%) had available perioperative data and were included in the analysis. The median (interquartile range [IQR]) age of recipients was 56 (49-62) years, and 1294 recipients (64.4%) were men. Overall survival and graft-failure-free survival rates were 83% and 81% at year 1, 74% and 71% at year 3, and 69% and 65% at year 5, with an 11.1% (222 recipients) incidence of 3-month graft failure or death. Multivariate factors associated with 3-month graft failure–free survival included post-LT aspartate aminotransferase level, international normalized ratio, bilirubin level, and platelet count, measures of which were used to calculate the Liver Graft Assessment Following Transplantation (L-GrAFT) risk score. The L-GrAFT model had an excellent C statistic of 0.85, with a significantly superior discrimination of 3-month graft failure–free survival compared with the existing EAD definition (C statistic, 0.68; P < .001) and the MEAF score (C statistic, 0.70; P < .001). Compared with patients with lower L-GrAFT risk, LT recipients in the highest 10th percentile of L-GrAFT scores had higher Model for End-Stage Liver Disease scores (median [IQR], 34 [26-40] vs 31 [25-38]; P = .005); greater need for pretransplant hospitalization (56.8% vs 44.8%; P = .003), renal replacement therapy (42.9% vs 30.5%; P < .001), mechanical ventilation (35.8% vs 18.1%; P < .001), and vasopressors (22.9% vs 11.0%; P < .001); longer cold ischemia times (median [IQR], 436 [311-539] vs 401 [302-506] minutes; P = .04); greater intraoperative blood transfusions (median [IQR], 17 [10-26] vs 10 [6-17] units of packed red blood cells; P < .001); and older donors (median [IQR] age, 47 [28-56] vs 41 [25-52] years; P < .001).
Conclusions and Relevance The L-GrAFT risk score allows a highly accurate, individualized risk estimation of 3-month graft failure following LT that is more accurate than existing EAD and MEAF scores. Multicenter validation may allow for the adoption of the L-GrAFT as a tool for evaluating the need for a retransplant, for establishing standardized grading of early allograft function across transplant centers, and as a highly accurate clinical end point in translational studies aiming to mitigate ischemia or reperfusion injury by modulating donor quality and recipient factors.
中文翻译:

使用移植风险评分模型的肝移植评估评估同种异体早期功能
重要性 肝移植(LT)后的早期同种异体移植功能障碍(EAD)明确预示着不良的移植物和患者预后,但缺乏广泛接受的分类或分级系统。
目的 建立个体化的LT术后移植失败风险评估模型,然后将其与现有二元EAD定义(术后第7天胆红素水平≥10mg / dL)的预后性能进行比较,术后国际标准化比值≥1.6 7,或在开始的7天内天冬氨酸转氨酶或丙氨酸转氨酶水平> 2000 U / L)和早期同种异体移植功能模型(MEAF)评分。
设计,设置和参加者 这项回顾性单中心分析使用了一个移植数据库,以识别所有接受过原发性LT并在David Geffen学校的Dumont-UCLA移植中心接受LT后10天实验室变量数据的成年患者。于2002年2月1日至2015年6月30日在加州大学洛杉矶分校获得医学博士学位。数据收集时间为2016年1月4日至2016年6月30日。数据分析时间为2016年7月1日至2017年8月30日。
主要结果和措施 三个月的无移植失败生存期。
结果 在研究期间接受原发性LT的2021例患者中,2008年(99.4%)具有可用的围手术期数据并被纳入分析。接受者的中位年龄(四分位数范围[IQR])为56(49-62)岁,男性为1294接受者(64.4%)。第1年的总生存率和无移植物存活率分别为83%和81%,第3年分别为74%和71%,第5年分别为69%和65%,发生率为31.1的11.1%(222位接受者)个月的移植失败或死亡。与3个月无移植失败存活率相关的多因素包括LT后天冬氨酸转氨酶水平,国际标准化比率,胆红素水平和血小板计数,这些指标均用于计算移植后肝移植评估(L-GrAFT)的风险分数。L-GrAFT模型的C统计量为0.85,P <.001)和MEAF评分(C统计量,0.70;P <.001)。与L-GrAFT风险较低的患者相比,L-GrAFT得分最高的10%的LT接受者具有较高的终末期肝病模型评分(中位[IQR],34 [26-40]和31 [25-38] ]; P = .005); 移植前住院的需求更大(56.8%vs 44.8%; P = .003),肾脏替代疗法(42.9%vs 30.5%; P <.001),机械通气(35.8%vs 18.1%; P <.001)和血管加压药(22.9%对11.0%; P <.001); 更长的冷缺血时间(中位[IQR],436 [311-539] vs 401 [302-506]分钟;P = .04); 术中输血量更大(中位[IQR],填充红细胞的单位为17 [10-26] vs 10 [6-17]单位;P <.001);和较年长的捐赠者([IQR]年龄中位数,分别为47岁[28-56]岁和41岁[25-52]岁;P <.001)。
结论和相关性 L-GrAFT风险评分可以对LT术后3个月的移植失败进行高度准确,个性化的风险评估,其准确性要高于现有的EAD和MEAF评分。多中心验证可能允许采用L-GrAFT作为评估重新移植需求的工具,用于跨移植中心建立早期同种异体移植功能的标准化等级,以及作为旨在减轻局部缺血的转化研究的高度准确的临床终点或通过调节供体质量和受者因素重新灌注损伤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号