当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Urea Crystal Dissolution in Water from Molecular Dynamics Simulation
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jpcb.7b07096 Abhinav Anand 1 , G. N. Patey 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jpcb.7b07096 Abhinav Anand 1 , G. N. Patey 1
Affiliation
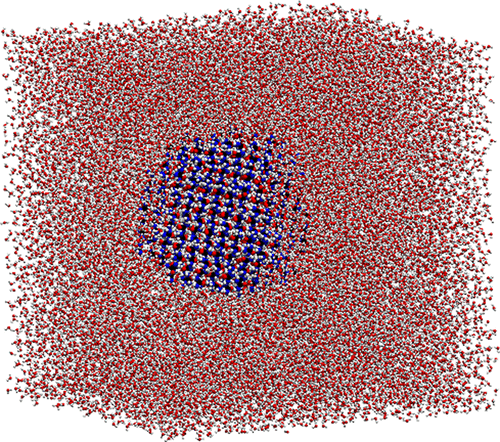
|
Molecular dynamics simulations are used to determine the mechanism of urea crystal dissolution in water under sink conditions. Crystals of cubic and tablet shapes are considered, and results are reported for four commonly used water models. The dissolution rates for different water models can differ considerably, but the overall dissolution mechanism remains the same. Urea dissolution occurs in three stages: a relatively fast initial stage, a slower intermediate stage, and a final stage. We show that the long intermediate stage is well described by classical rate laws, which assume that the dissolution rate is proportional to the active surface area. By carrying out simulations at different temperatures, we show that urea dissolution is an activated process, with an activation energy of ∼32 kJ mol–1. Our simulations give no indication of a significant diffusion layer, and we conclude that the detachment of molecules from the crystal is the rate-determining step for dissolution. The results we report for urea are consistent with earlier observations for the dissolution of NaCl crystals. This suggests that the three-stage mechanism and classical rate laws might apply to the dissolution of other ionic and molecular crystals.
中文翻译:

基于分子动力学模拟的尿素晶体在水中的溶解机理
分子动力学模拟用于确定水槽条件下尿素晶体在水中的溶解机理。考虑了立方和片状晶体,并报告了四种常用水模型的结果。不同水模型的溶出速率可能有很大差异,但总体溶出机理保持不变。尿素溶解分为三个阶段:相对较快的初始阶段,较慢的中间阶段和最后阶段。我们表明,经典的速率定律很好地描述了较长的中间阶段,该定律假设溶出速率与活性表面积成正比。通过在不同温度下进行模拟,我们发现尿素溶解是一个活化过程,活化能约为32 kJ mol –1。我们的模拟没有给出明显的扩散层的迹象,并且我们得出结论,分子与晶体的分离是溶解的决定速率的步骤。我们报告的尿素结果与NaCl晶体溶解的早期观察结果一致。这表明三步机理和经典速率定律可能适用于其他离子和分子晶体的溶解。
更新日期:2018-01-08
中文翻译:

基于分子动力学模拟的尿素晶体在水中的溶解机理
分子动力学模拟用于确定水槽条件下尿素晶体在水中的溶解机理。考虑了立方和片状晶体,并报告了四种常用水模型的结果。不同水模型的溶出速率可能有很大差异,但总体溶出机理保持不变。尿素溶解分为三个阶段:相对较快的初始阶段,较慢的中间阶段和最后阶段。我们表明,经典的速率定律很好地描述了较长的中间阶段,该定律假设溶出速率与活性表面积成正比。通过在不同温度下进行模拟,我们发现尿素溶解是一个活化过程,活化能约为32 kJ mol –1。我们的模拟没有给出明显的扩散层的迹象,并且我们得出结论,分子与晶体的分离是溶解的决定速率的步骤。我们报告的尿素结果与NaCl晶体溶解的早期观察结果一致。这表明三步机理和经典速率定律可能适用于其他离子和分子晶体的溶解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号