当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Study of Uracil Tautomeric Forms in the Ribosome: The Case of Uracil and 5-Oxyacetic Acid Uracil in the First Anticodon Position of tRNA
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b10878 Yossa Dwi Hartono 1, 2 , Mika Ito 1 , Alessandra Villa 1 , Lennart Nilsson 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b10878 Yossa Dwi Hartono 1, 2 , Mika Ito 1 , Alessandra Villa 1 , Lennart Nilsson 1
Affiliation
|
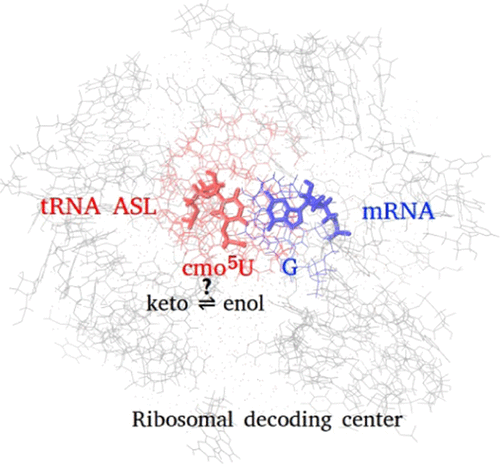
Tautomerism is important in many biomolecular interactions, not least in RNA biology. Crystallographic studies show the possible presence of minor tautomer forms of transfer-RNA (tRNA) anticodon bases in the ribosome. The hydrogen positions are not resolved in the X-ray studies, and we have used ab initio calculations and molecular dynamics simulations to understand if and how the minor enol form of uracil (U), or the modified uracil 5-oxyacetic acid (cmo5U), can be accommodated in the tRNA–messenger-RNA interactions in the ribosome decoding center. Ab initio calculations on isolated bases show that the modification affects the keto–enol equilibrium of the uracil base only slightly; the keto form is dominant (>99.99%) in both U and cmo5U. Other factors such as interactions with the surrounding nucleotides or ions would be required to shift the equilibrium toward the enol tautomer. Classical molecular simulations show a better agreement with the X-ray structures for the enol form, but free energy calculations indicate that the most stable form is the keto. In the ribosome, the enol tautomers of U and cmo5U pair with a guanine forming two hydrogen bonds, which do not involve the enol group. The oxyacetic acid modification has a minor effect on the keto–enol equilibrium.
中文翻译:

核糖体中尿嘧啶互变异构形式的计算研究:尿嘧啶和5-氧乙酸尿嘧啶在tRNA的第一个反密码子位置的情况
互变异构在许多生物分子相互作用中很重要,尤其是在RNA生物学中。晶体学研究表明,核糖体中可能存在次要互变异构体形式的转移RNA(tRNA)反密码子碱基。在X射线研究中无法解析氢的位置,我们使用了从头算和分子动力学模拟来了解是否以及如何使次要烯醇形式的尿嘧啶(U)或修饰的尿嘧啶5-氧乙酸(cmo 5 U),可以容纳在核糖体解码中心的tRNA-信使-RNA相互作用中。对孤立碱基的从头算计算表明,修饰仅对尿嘧啶碱基的酮-烯醇平衡产生轻微影响;酮形式在U和cmo 5中均占主导地位(> 99.99%)U.需要其他因素,例如与周围核苷酸或离子的相互作用,以使平衡向烯醇互变异构体转移。经典的分子模拟表明,烯醇形式与X射线结构具有更好的一致性,但自由能计算表明,最稳定的形式是酮基。在核糖体中,U和cmo 5 U的烯醇互变异构体与鸟嘌呤成对,形成两个不涉及烯醇基团的氢键。氧乙酸修饰对酮-烯醇平衡的影响较小。
更新日期:2018-01-11
中文翻译:

核糖体中尿嘧啶互变异构形式的计算研究:尿嘧啶和5-氧乙酸尿嘧啶在tRNA的第一个反密码子位置的情况
互变异构在许多生物分子相互作用中很重要,尤其是在RNA生物学中。晶体学研究表明,核糖体中可能存在次要互变异构体形式的转移RNA(tRNA)反密码子碱基。在X射线研究中无法解析氢的位置,我们使用了从头算和分子动力学模拟来了解是否以及如何使次要烯醇形式的尿嘧啶(U)或修饰的尿嘧啶5-氧乙酸(cmo 5 U),可以容纳在核糖体解码中心的tRNA-信使-RNA相互作用中。对孤立碱基的从头算计算表明,修饰仅对尿嘧啶碱基的酮-烯醇平衡产生轻微影响;酮形式在U和cmo 5中均占主导地位(> 99.99%)U.需要其他因素,例如与周围核苷酸或离子的相互作用,以使平衡向烯醇互变异构体转移。经典的分子模拟表明,烯醇形式与X射线结构具有更好的一致性,但自由能计算表明,最稳定的形式是酮基。在核糖体中,U和cmo 5 U的烯醇互变异构体与鸟嘌呤成对,形成两个不涉及烯醇基团的氢键。氧乙酸修饰对酮-烯醇平衡的影响较小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号