当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Organocatalytic Approach to 2,4-Disubstituted 1,2,3-Triazoles by N2-Selective Aza-Michael Addition
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02793 Ujjawal Kumar Bhagat 1 , Rama Krishna Peddinti 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02793 Ujjawal Kumar Bhagat 1 , Rama Krishna Peddinti 1
Affiliation
|
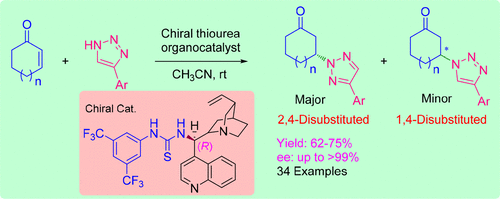
Despite the fact that N1-functionalization of NH-1,2,3-triazoles has been known for several decades, enantioselective variants of this reaction have only recently been developed. Nevertheless, functionalization at the N2-position of NH-1,2,3-triazoles leading to optically active N2-substituted triazoles is not yet developed. In this article, we report, for the first time, the asymmetric aza-Michael reaction of 4-aryl-NH-1,2,3-triazoles to cyclic enones under the catalytic influence of chiral bifunctional thiourea organocatalysts for the enantioselective generation of 2,4-disubstituted 1,2,3-triazoles. The cinchonine-derived thiourea catalyst III worked efficiently in the current transformation to produce N2-functionalized 1,2,3-triazoles as major products in optical yields up to >99.9% along with minor 1,4-disubstitued 1,2,3-triazoles.
中文翻译:

N2-选择性氮杂-迈克尔加成反应对2,4-二取代的1,2,3-三唑的不对称有机催化方法
尽管N个N1-官能^ h -1,2,3-三唑已经知道了几十年,这种反应的对映选择性变种最近才开发的。然而,尚未开发出在N H -1,2,3-三唑的N2-位上的官能化导致光学活性的N2-取代的三唑。在本文中,我们首次报道了在手性双官能硫脲有机催化剂的催化作用下,4-芳基-N H -1,2,3-三唑与环烯酮的不对称氮杂-迈克尔反应,生成对映体。 2,4-二取代的1,2,3-三唑。辛可宁衍生的硫脲催化剂III 在当前的转换中有效地工作,以生产N2官能化的1,2,3-三唑为主要产品,光学收率高达> 99.9%,以及次要的1,4-二取代的1,2,3-三唑。
更新日期:2018-01-10
中文翻译:

N2-选择性氮杂-迈克尔加成反应对2,4-二取代的1,2,3-三唑的不对称有机催化方法
尽管N个N1-官能^ h -1,2,3-三唑已经知道了几十年,这种反应的对映选择性变种最近才开发的。然而,尚未开发出在N H -1,2,3-三唑的N2-位上的官能化导致光学活性的N2-取代的三唑。在本文中,我们首次报道了在手性双官能硫脲有机催化剂的催化作用下,4-芳基-N H -1,2,3-三唑与环烯酮的不对称氮杂-迈克尔反应,生成对映体。 2,4-二取代的1,2,3-三唑。辛可宁衍生的硫脲催化剂III 在当前的转换中有效地工作,以生产N2官能化的1,2,3-三唑为主要产品,光学收率高达> 99.9%,以及次要的1,4-二取代的1,2,3-三唑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号