当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
OsXCl(phosphine)2(diamine) and OsXCl(diphosphine)(diamine) (X = Cl, H) Complexes for Ketone Hydrogenation
Organometallics ( IF 2.5 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00737 Cinzia Barbato 1 , Salvatore Baldino 1 , Maurizio Ballico 1 , Rosario Figliolia 1 , Santo Magnolia 1 , Katia Siega 1 , Eberhardt Herdtweck 2 , Paolo Strazzolini 1 , Giorgio Chelucci 3 , Walter Baratta 1
Organometallics ( IF 2.5 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00737 Cinzia Barbato 1 , Salvatore Baldino 1 , Maurizio Ballico 1 , Rosario Figliolia 1 , Santo Magnolia 1 , Katia Siega 1 , Eberhardt Herdtweck 2 , Paolo Strazzolini 1 , Giorgio Chelucci 3 , Walter Baratta 1
Affiliation
|
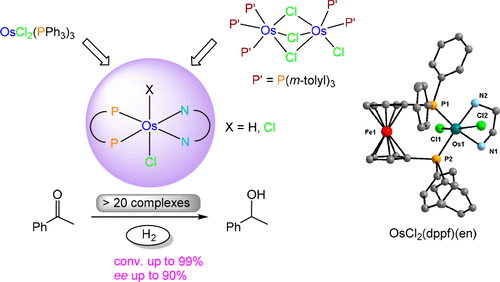
The osmium complex trans-[OsCl2(PPh3)2(en)] (2) was prepared by reaction of [OsCl2(PPh3)3] (1a) with ethylenediamine (en), whereas the diphosphine derivatives trans-[OsCl2(dppf)(NN)] (NN = en (3), bn (4; bn = 1,4-butanediamine)) and trans-[OsCl2(dpbp)(en)] (5) were obtained from 1a, dppf or dpbp, and the corresponding NN ligand in CH2Cl2 or toluene. An X-ray diffraction study has been provided for 3. The isolation of the chiral derivatives trans-[OsCl2(diphosphine)((R,R)-dpen)] (diphosphine = dppf (6), dpbp (7), (R,R)-skewphos (8)) was achieved by reacting 1a with the diphosphine and (R,R)-dpen in toluene. Treatment of the precursor [Os2Cl4(P(m-tolyl)3)5] (1b) with en afforded [OsCl2(P(m-tolyl)3)2(en)] (9), while reaction of 1b with dppb and N,N-dmen gave [OsCl2(dppb)(N,N-dmen)] (10). The chiral derivatives [OsCl2(diphosphine)(NN)] (11–21; diphosphine = (S)-MeObiphep, (R)-MeObiphep, (R)-xylMeObiphep, (R)-binap, (S)-xylbinap, (R)-xylbinap, (R,S)-Josiphos*; NN = en, (R,R)-dpen, (R)-daipen, (R,R)-dppn) were prepared from 1b and the corresponding diphosphine and NN ligands in toluene. The monohydride trans-[OsHCl(P(m-tolyl)3)2(en)] (22) was synthesized by reaction of 1b with H2 (1 atm) in the presence of NEt3, followed by addition of en in toluene. Similarly, trans-[OsHCl(dppf)(en)] (23) was synthesized from 1a, H2, and NEt3, followed by treatment with dppf and en. Complexes 2–5, 9, 10, 22, and 23 efficiently catalyzed the hydrogenation of acetophenone with H2 under low pressure (5 atm) at 60–70 °C in ethanol (1–2 mol % of NaOEt) with the ratio S/C = 5000–10000. The chiral derivatives 6–8 and 11–21 afforded the asymmetric hydrogenation of acetophenone with up to 90% ee by combining bulky xylyl-substituted MeObiphep or binap-type ligands with (R)-daipen or (R,R)-dpen ligands. Catalytic transfer hydrogenation of acetophenone was observed with 3, 6, and 7 (S/C = 2000) in 2-propanol and in the presence of NaOiPr (2 mol %) at 60–82 °C.
中文翻译:

用于酮加氢的OsXCl(膦)2(二胺)和OsXCl(二膦)(二胺)(X = Cl,H)配合物
通过[OsCl 2(PPh 3)3 ](1a)与乙二胺(en)反应制得O络合物反式-[OsCl 2(PPh 3)2(en)](2),而二膦衍生物反式-[[ OSCL 2(DPPF)(NN)](NN = EN(3),BN(4 ; BN = 1,4-丁二胺))和反式- [OSCL 2(DPBP)(烯)](5从获得)1A,dppf或dpbp,以及CH 2 Cl 2中相应的NN配体或甲苯。X射线衍射研究已经提供了3。分离了手性衍生物反式-[OsCl 2(diphosphine)((R,R)-dpen)](diphosphine = dppf(6),dpbp(7),(R,R)-skewphos(8))使1a与二膦和(R,R)-dpen在甲苯中反应。所述前体[Os中治疗2氯4(P(米间-甲苯基)3)5 ](图1b)带连接,得到[OSCL2(P(米间-甲苯基)3)2(烯)](9),而反应1B与DPPB和Ñ,Ñ -dmen得到[OSCL 2(DPPB)(Ñ,Ñ -dmen)](10)。手性衍生物〔OSCL 2(二膦)(NN)](11 - 21 ;二膦=(小号)-MeOBIPHEP,(- [R)-MeOBIPHEP,(- [R)-xylMeObiphep,(- [R)-BINAP,(小号)-xylbinap, (R)-木基联萘酚,(R,S)-Josiphos *; 由1b以及甲苯中的相应二膦和NN配体制备NN = en,(R,R)-dpen,(R)-daipen,(R,R)-dppn)。通过在NEt 3存在下1b与H 2(1 atm)反应,然后在甲苯中添加en,合成一价氢化物反式-[OsHCl(P(m- tolyl)3)2(en)](22)。同样,反式-[OsHCl(dppf)(en)](23)由1a,H 2和NEt 3合成,然后用dppf和en处理。配合物2 - 5,9,10,22,和23有效地催化苯乙酮的氢化反应用H 2在60〜70℃下,在乙醇(1-2摩尔%的NaOEt)与所述比率S低压(5大气压)下/ C = 5000–10000。手性衍生物6 – 8和11 – 21通过将庞大的二甲苯基取代的MeObiphep或binap型配体与(R)-daipen或(R,R)-dpen配体。用观察到的苯乙酮的催化转移氢化3,6,和7(S / C = 2000)在2-丙醇和NaO等的存在下我在60-82℃下PR(2摩尔%)。
更新日期:2017-12-20
中文翻译:

用于酮加氢的OsXCl(膦)2(二胺)和OsXCl(二膦)(二胺)(X = Cl,H)配合物
通过[OsCl 2(PPh 3)3 ](1a)与乙二胺(en)反应制得O络合物反式-[OsCl 2(PPh 3)2(en)](2),而二膦衍生物反式-[[ OSCL 2(DPPF)(NN)](NN = EN(3),BN(4 ; BN = 1,4-丁二胺))和反式- [OSCL 2(DPBP)(烯)](5从获得)1A,dppf或dpbp,以及CH 2 Cl 2中相应的NN配体或甲苯。X射线衍射研究已经提供了3。分离了手性衍生物反式-[OsCl 2(diphosphine)((R,R)-dpen)](diphosphine = dppf(6),dpbp(7),(R,R)-skewphos(8))使1a与二膦和(R,R)-dpen在甲苯中反应。所述前体[Os中治疗2氯4(P(米间-甲苯基)3)5 ](图1b)带连接,得到[OSCL2(P(米间-甲苯基)3)2(烯)](9),而反应1B与DPPB和Ñ,Ñ -dmen得到[OSCL 2(DPPB)(Ñ,Ñ -dmen)](10)。手性衍生物〔OSCL 2(二膦)(NN)](11 - 21 ;二膦=(小号)-MeOBIPHEP,(- [R)-MeOBIPHEP,(- [R)-xylMeObiphep,(- [R)-BINAP,(小号)-xylbinap, (R)-木基联萘酚,(R,S)-Josiphos *; 由1b以及甲苯中的相应二膦和NN配体制备NN = en,(R,R)-dpen,(R)-daipen,(R,R)-dppn)。通过在NEt 3存在下1b与H 2(1 atm)反应,然后在甲苯中添加en,合成一价氢化物反式-[OsHCl(P(m- tolyl)3)2(en)](22)。同样,反式-[OsHCl(dppf)(en)](23)由1a,H 2和NEt 3合成,然后用dppf和en处理。配合物2 - 5,9,10,22,和23有效地催化苯乙酮的氢化反应用H 2在60〜70℃下,在乙醇(1-2摩尔%的NaOEt)与所述比率S低压(5大气压)下/ C = 5000–10000。手性衍生物6 – 8和11 – 21通过将庞大的二甲苯基取代的MeObiphep或binap型配体与(R)-daipen或(R,R)-dpen配体。用观察到的苯乙酮的催化转移氢化3,6,和7(S / C = 2000)在2-丙醇和NaO等的存在下我在60-82℃下PR(2摩尔%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号