当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nature and Value of Freely Dissolved EPS Ecosystem Services: Insight into Molecular Coupling Mechanisms for Regulating Metal Toxicity
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.est.7b04834 Weijun Shou 1 , Fuxing Kang 1 , Jiahao Lu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.est.7b04834 Weijun Shou 1 , Fuxing Kang 1 , Jiahao Lu 1
Affiliation
|
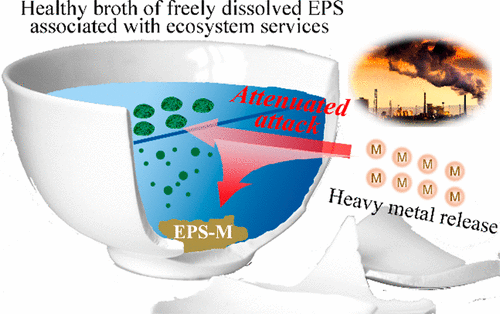
Extracellular polymeric substances (EPSs) dispersed in natural waters play a significant role in relieving impacts to microbial survival associated with heavy metal release, yet little is known about the association of freely dissolved EPS ecosystem services with metal transformation in natural waters. Here, we demonstrate that dispersive EPSs mitigate the metal toxicity to microbial cells through an associative coordination reaction. Microtitrimetry coupled with fluorescence spectroscopy ascribes the combination of freely dissolved EPSs from Escherichia coli (E. coli) with Cu2+/Cd2+ to a coordination reaction associated with chemical static quenching. Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and computational chemistry confirm that carboxyl residues in protein-like substances of the EPSs are responsible for the coordination. Frontier molecular orbitals (MOs) of a deprotonated carboxyl integrate with the occupied d orbitals of Cu2+ and/or d, s orbitals of Cd2+ to form metal-EPS complexes. Microcosmic systems show that because the metal-EPS complexes decrease cellular absorbability of metals, E. coli survivals increase by 4.3 times for Cu2+ and 1.6 times for Cd2+, respectively. Based on bonding energies for six metals-EPS coordination, an associative toxic effect further confirms that increased bonding energies facilitate retardation of metals in the EPS matrix, protecting against E. coli apoptosis.
中文翻译:

可自由溶解的EPS生态系统服务的性质和价值:调节金属毒性的分子偶联机制的见解
分散在天然水中的细胞外聚合物质(EPS)在缓解与重金属释放有关的微生物生存的影响方面起着重要作用,但人们对游离溶解的EPS生态系统服务与天然水中的金属转化之间的关系知之甚少。在这里,我们证明了分散EPS可以通过缔合配位反应减轻金属对微生物细胞的毒性。微量滴定结合荧光光谱法将大肠杆菌(E. coli)中自由溶解的EPS与Cu 2+ / Cd 2+结合起来使用与化学静态猝灭有关的配位反应。傅里叶变换红外光谱(FTIR),X射线光电子能谱(XPS)和计算化学证实,EPS的蛋白样物质中的羧基残基负责配位。去质子化的羧基的前沿分子轨道(MOs)与Cu 2+的占据d轨道和/或Cd 2+的d,s轨道结合形成金属EPS配合物。微观系统表明,由于金属-EPS配合物降低了金属对细胞的吸收能力,因此,Cu 2+的大肠杆菌存活率增加了4.3倍,Cd 2+的大肠杆菌存活率增加了1.6倍, 分别。基于六种金属与EPS配位的结合能,缔合的毒性效应进一步证实,增加的结合能有助于EPS基质中金属的阻滞,从而防止大肠杆菌凋亡。
更新日期:2018-01-06
中文翻译:

可自由溶解的EPS生态系统服务的性质和价值:调节金属毒性的分子偶联机制的见解
分散在天然水中的细胞外聚合物质(EPS)在缓解与重金属释放有关的微生物生存的影响方面起着重要作用,但人们对游离溶解的EPS生态系统服务与天然水中的金属转化之间的关系知之甚少。在这里,我们证明了分散EPS可以通过缔合配位反应减轻金属对微生物细胞的毒性。微量滴定结合荧光光谱法将大肠杆菌(E. coli)中自由溶解的EPS与Cu 2+ / Cd 2+结合起来使用与化学静态猝灭有关的配位反应。傅里叶变换红外光谱(FTIR),X射线光电子能谱(XPS)和计算化学证实,EPS的蛋白样物质中的羧基残基负责配位。去质子化的羧基的前沿分子轨道(MOs)与Cu 2+的占据d轨道和/或Cd 2+的d,s轨道结合形成金属EPS配合物。微观系统表明,由于金属-EPS配合物降低了金属对细胞的吸收能力,因此,Cu 2+的大肠杆菌存活率增加了4.3倍,Cd 2+的大肠杆菌存活率增加了1.6倍, 分别。基于六种金属与EPS配位的结合能,缔合的毒性效应进一步证实,增加的结合能有助于EPS基质中金属的阻滞,从而防止大肠杆菌凋亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号