当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Differential Binding Affinities and Allosteric Conformational Changes Underlie Interactions of Yorkie and a Multivalent PPxY Partner
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.biochem.7b00973 Afua Nyarko 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.biochem.7b00973 Afua Nyarko 1
Affiliation
|
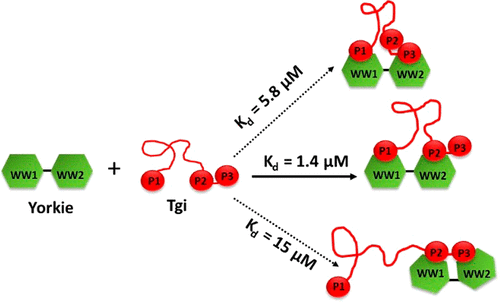
Tondu domain-containing growth inhibitor (Tgi) is one of a growing number of multivalent PPxY proteins that regulate cell growth via interactions with the tandem WW domains of the transcription coactivator protein, Yorkie (Yki). These proteins are attractive candidates for targeted drug design, but the substantial amount of disorder predicted from their primary sequences makes structural studies that are foundational to drug design challenging. We have successfully overexpressed full length recombinant Tgi and Yki, experimentally confirmed that intrinsic structural disorder is common to both proteins, and assessed binding of the Yki WW domains to the three Tgi PPxY motifs using nuclear magnetic resonance and isothermal titration calorimetry. We find that the tandem WW domains positively cooperate to engage all three PPxY sites with a broad range of affinities. The first PPxY motif that is quite distant from the other two serves as the “binding initiation” site and is essential for high-affinity interactions. Importantly, by monitoring binding to the full length or larger protein domains, we obtain more physiologically relevant affinity information and identify “long-range” residues that could be targeted to fine-tune binding. This expansion of protein functionality through modulation of residues outside the recognition sequences offers potential alternative targets for drug design.
中文翻译:

差异绑定亲和力和变构构象变化是约克和一个多价PPxY伙伴相互作用的基础。
含Tondu结构域的生长抑制剂(Tgi)是越来越多的多价PPxY蛋白之一,该蛋白通过与转录辅助激活蛋白Yorkyie(Yki)的串联WW结构域的相互作用来调节细胞的生长。这些蛋白质是靶向药物设计的诱人候选物,但从其一级序列预测的大量疾病使结构研究成为药物设计的基础。我们已经成功地过表达了全长重组Tgi和Yki,通过实验证实了内在结构紊乱是两种蛋白质共有的,并使用核磁共振和等温滴定量热法评估了Yki WW结构域与三个Tgi PPxY基序的结合。我们发现串联WW域积极合作,以广泛的亲和力参与了所有三个PPxY网站。与其他两个序列相距甚远的第一个PPxY基序充当“结合起始”位点,对于高亲和力相互作用至关重要。重要的是,通过监测与全长或更大蛋白结构域的结合,我们获得了更多与生理相关的亲和力信息,并确定了可用于微调结合的“远距离”残基。通过调节识别序列外的残基来扩展蛋白质功能,为药物设计提供了潜在的替代靶标。通过监测与全长或更大蛋白结构域的结合,我们获得了更多与生理相关的亲和力信息,并确定了可用于微调结合的“远距离”残基。通过调节识别序列外的残基来扩展蛋白质功能,为药物设计提供了潜在的替代靶标。通过监测与全长或更大蛋白结构域的结合,我们获得了更多与生理相关的亲和力信息,并确定了可用于微调结合的“远距离”残基。通过调节识别序列外的残基来扩展蛋白质功能,为药物设计提供了潜在的替代靶标。
更新日期:2018-01-05
中文翻译:

差异绑定亲和力和变构构象变化是约克和一个多价PPxY伙伴相互作用的基础。
含Tondu结构域的生长抑制剂(Tgi)是越来越多的多价PPxY蛋白之一,该蛋白通过与转录辅助激活蛋白Yorkyie(Yki)的串联WW结构域的相互作用来调节细胞的生长。这些蛋白质是靶向药物设计的诱人候选物,但从其一级序列预测的大量疾病使结构研究成为药物设计的基础。我们已经成功地过表达了全长重组Tgi和Yki,通过实验证实了内在结构紊乱是两种蛋白质共有的,并使用核磁共振和等温滴定量热法评估了Yki WW结构域与三个Tgi PPxY基序的结合。我们发现串联WW域积极合作,以广泛的亲和力参与了所有三个PPxY网站。与其他两个序列相距甚远的第一个PPxY基序充当“结合起始”位点,对于高亲和力相互作用至关重要。重要的是,通过监测与全长或更大蛋白结构域的结合,我们获得了更多与生理相关的亲和力信息,并确定了可用于微调结合的“远距离”残基。通过调节识别序列外的残基来扩展蛋白质功能,为药物设计提供了潜在的替代靶标。通过监测与全长或更大蛋白结构域的结合,我们获得了更多与生理相关的亲和力信息,并确定了可用于微调结合的“远距离”残基。通过调节识别序列外的残基来扩展蛋白质功能,为药物设计提供了潜在的替代靶标。通过监测与全长或更大蛋白结构域的结合,我们获得了更多与生理相关的亲和力信息,并确定了可用于微调结合的“远距离”残基。通过调节识别序列外的残基来扩展蛋白质功能,为药物设计提供了潜在的替代靶标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号