当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Artificial Photosynthesis for Formaldehyde Production with 85% of Faradaic Efficiency by Tuning the Reduction Potential
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acscatal.7b02953 Chang Woo Kim 1, 2 , Myung Jong Kang 1 , Sohyun Ji 1 , Young Soo Kang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acscatal.7b02953 Chang Woo Kim 1, 2 , Myung Jong Kang 1 , Sohyun Ji 1 , Young Soo Kang 1
Affiliation
|
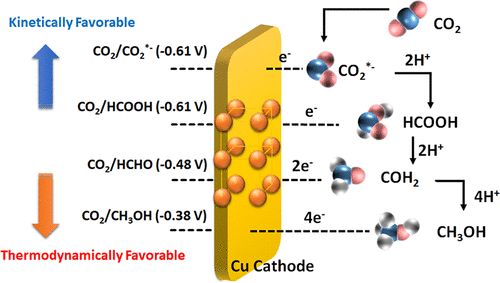
In the present study, artificial photosynthesis by CO2 reduction in NaCl is studied for solar fuel production. C1 fuels are evolved from various photoelectrochemical reactions at different reduction potentials depending on the multistep electron-transfer processes to CO2. The (040) facet engineered BiVO4 (040-BVO)|NaCl|Cu system is illuminated with solar light, and the external bias potential is tuned from 0.7 V to 1.5 (vs RHE) for the CO2 reduction reaction. Integrating the applied external bias potential into the conduction band minimum of 040-BVO enables the CO2 molecules to be converted into valuable chemical fuels. This thermodynamic control leads to product selectivity and increased faradaic efficiency. We observed that the selectivity and yield of the products depend on the magnitude of the CO2 reduction potential. Its products were obtained by tuning the appropriate reduction potential, leading to faradaic efficiencies of 62% for formic acid, 85% for formaldehyde, 8% for MeOH, and 6% for EtOH at different bias in NaCl electrolyte. The correlation between the production of solar chemical fuels and CO2 reduction potential tuning was studied by applying an external bias potential on 040-BVO|NaCl|Cu. The Cu–C bond distance change during photoelectrochemical CO2 reduction reaction was investigated with in situ synchrotron X-ray. We suggest that the present results represent the most viable strategy to selectively and efficiently produce solar fuels via artificial photosynthesis.
中文翻译:

通过调节还原电位,人工光合作用生产法拉第效率的85%的甲醛
在本研究中,研究了通过在NaCl中还原CO 2进行人工光合作用来生产太阳能。C1燃料是由不同的光电化学反应以不同的还原电势演化而来的,具体取决于将多步电子转移至CO 2的过程。(040)刻面工程化的BiVO 4(040-BVO)| NaCl | Cu系统用太阳光照射,并且外部偏置电势从0.7 V调整为1.5(vs RHE)以进行CO 2还原反应。将施加的外部偏置电势整合到040-BVO的最小导带中,即可实现CO 2分子将转化为有价值的化学燃料。这种热力学控制导致产物选择性和法拉第效率的提高。我们观察到产物的选择性和产率取决于CO 2还原潜力的大小。通过调节适当的还原电位可得到其产品,在不同的NaCl电解液中,法拉第效率为甲酸的62%,甲醛的85%,MeOH的8%和EtOH的法拉第效率。通过在040-BVO | NaCl | Cu上施加外部偏置电势,研究了太阳能化学燃料的生产与CO 2还原电势调整之间的相关性。在光电化学CO 2中Cu–C键的距离变化用原位同步加速器X射线研究还原反应。我们建议,目前的结果代表了通过人工光合作用有选择地和有效地生产太阳能的最可行策略。
更新日期:2018-01-05
中文翻译:

通过调节还原电位,人工光合作用生产法拉第效率的85%的甲醛
在本研究中,研究了通过在NaCl中还原CO 2进行人工光合作用来生产太阳能。C1燃料是由不同的光电化学反应以不同的还原电势演化而来的,具体取决于将多步电子转移至CO 2的过程。(040)刻面工程化的BiVO 4(040-BVO)| NaCl | Cu系统用太阳光照射,并且外部偏置电势从0.7 V调整为1.5(vs RHE)以进行CO 2还原反应。将施加的外部偏置电势整合到040-BVO的最小导带中,即可实现CO 2分子将转化为有价值的化学燃料。这种热力学控制导致产物选择性和法拉第效率的提高。我们观察到产物的选择性和产率取决于CO 2还原潜力的大小。通过调节适当的还原电位可得到其产品,在不同的NaCl电解液中,法拉第效率为甲酸的62%,甲醛的85%,MeOH的8%和EtOH的法拉第效率。通过在040-BVO | NaCl | Cu上施加外部偏置电势,研究了太阳能化学燃料的生产与CO 2还原电势调整之间的相关性。在光电化学CO 2中Cu–C键的距离变化用原位同步加速器X射线研究还原反应。我们建议,目前的结果代表了通过人工光合作用有选择地和有效地生产太阳能的最可行策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号