当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of σ,π-Digold(I) Alkyne Complexes in Reactions of Enynes.
Organometallics ( IF 2.5 ) Pub Date : 2017-12-19 , DOI: 10.1021/acs.organomet.7b00668 Sofia Ferrer 1 , Antonio M Echavarren 1, 2
Organometallics ( IF 2.5 ) Pub Date : 2017-12-19 , DOI: 10.1021/acs.organomet.7b00668 Sofia Ferrer 1 , Antonio M Echavarren 1, 2
Affiliation
|
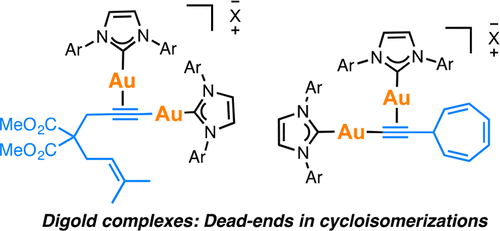
Gold(I) acetylide and σ,π-digold(I) alkyne complexes derived from one prototypical 1,6-enyne and from 7-ethynyl-1,3,5-cycloheptatriene have been prepared and structurally characterized. Their possible role in gold(I)-catalyzed cycloisomerizations has been studied by experiment and by DFT calculations. Gold(I) acetylides are totally unproductive complexes in the absence of Brønsted acids. Similarly, no cyclizations were observed by heating σ,π-digold(I) alkyne digold(I) at least up to 130 °C. Theoretical studies provide a rationale for the much lower reactivity of digold species in reactions of enynes.
中文翻译:

σ,π-Digold(I)炔烃配合物在烯炔反应中的作用。
制备了乙炔化金(I)和衍生自一种原型1,6-烯炔和7-乙炔基1,3,5-环庚三烯的σ,π-乙二醛(I)炔配合物,并对其结构进行了表征。通过实验和DFT计算研究了它们在金(I)催化的环异构化中的可能作用。在不存在布朗斯台德酸的情况下,乙炔金(I)完全是无用的络合物。同样,通过加热σ,π-digold(I)炔digold(I)至少达到130°C,也未观察到环化反应。理论研究为乙炔物种在烯炔反应中的低得多的反应性提供了理论依据。
更新日期:2017-12-19
中文翻译:

σ,π-Digold(I)炔烃配合物在烯炔反应中的作用。
制备了乙炔化金(I)和衍生自一种原型1,6-烯炔和7-乙炔基1,3,5-环庚三烯的σ,π-乙二醛(I)炔配合物,并对其结构进行了表征。通过实验和DFT计算研究了它们在金(I)催化的环异构化中的可能作用。在不存在布朗斯台德酸的情况下,乙炔金(I)完全是无用的络合物。同样,通过加热σ,π-digold(I)炔digold(I)至少达到130°C,也未观察到环化反应。理论研究为乙炔物种在烯炔反应中的低得多的反应性提供了理论依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号