当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Measurement of the Differential Capacitance of Solvent-Free and Dilute Ionic Liquids.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-12-20 , DOI: 10.1021/acs.jpclett.7b02946 Monchai Jitvisate 1 , James R T Seddon 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-12-20 , DOI: 10.1021/acs.jpclett.7b02946 Monchai Jitvisate 1 , James R T Seddon 2
Affiliation
|
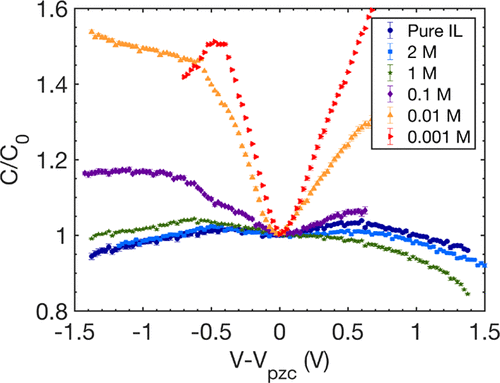
Differential capacitance is a key quantity in the understanding of electrical double-layer charging of electrolytes. However, experimental observations of ionic liquid systems are controversial, inconsistent, and often unable of confirming or refuting existing theories as well as highlighting discrepancies between the measurement techniques. We study the differential capacitance in both pure and dilute ionic liquids at room temperature. Using chronoamperometry to measure the differential capacitance of the liquids at a polycrystalline platinum electrode, we find good agreement between the measured capacitance curves and the extended mean-field model of Goodwin-Kornyshev [Goodwin, Z. A.; et al. Electrochim. Acta. 2017, 225, 190-197]. A crossover is found from the pure to the dilute regime, as shown by a transition from a camel-shape capacitance curve to a U-like one, together with a nonmonotonic dependence of capacitance with electrolyte concentration.
中文翻译:

无溶剂和稀离子液体的微分电容的直接测量。
差分电容是理解电解质的双电层电荷的关键因素。然而,对离子液体系统的实验观察是有争议的,前后不一致的,并且通常无法确认或反驳现有理论,也无法强调测量技术之间的差异。我们研究了室温下纯离子液体和稀离子液体中的差分电容。使用计时电流法测量多晶铂电极上液体的差分电容,我们发现测得的电容曲线与Goodwin-Kornyshev [Goodwin,ZA; 等。Electrochim。Acta。2017,225,190-197]。发现了从纯政权到稀政权的交叉,
更新日期:2017-12-20
中文翻译:

无溶剂和稀离子液体的微分电容的直接测量。
差分电容是理解电解质的双电层电荷的关键因素。然而,对离子液体系统的实验观察是有争议的,前后不一致的,并且通常无法确认或反驳现有理论,也无法强调测量技术之间的差异。我们研究了室温下纯离子液体和稀离子液体中的差分电容。使用计时电流法测量多晶铂电极上液体的差分电容,我们发现测得的电容曲线与Goodwin-Kornyshev [Goodwin,ZA; 等。Electrochim。Acta。2017,225,190-197]。发现了从纯政权到稀政权的交叉,











































 京公网安备 11010802027423号
京公网安备 11010802027423号