当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of novel diarylpyrimidines as potent HIV-1 NNRTIs by investigating the chemical space of a less explored “hydrophobic channel”
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7ob02828h Zhongxia Zhou 1, 2, 3, 4, 5 , Tao Liu 1, 2, 3, 4, 5 , Dongwei Kang 1, 2, 3, 4, 5 , Zhipeng Huo 1, 2, 3, 4, 5 , Gaochan Wu 1, 2, 3, 4, 5 , Dirk Daelemans 6, 7, 8, 9 , Erik De Clercq 6, 7, 8, 9 , Christophe Pannecouque 6, 7, 8, 9 , Peng Zhan 1, 2, 3, 4, 5 , Xinyong Liu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7ob02828h Zhongxia Zhou 1, 2, 3, 4, 5 , Tao Liu 1, 2, 3, 4, 5 , Dongwei Kang 1, 2, 3, 4, 5 , Zhipeng Huo 1, 2, 3, 4, 5 , Gaochan Wu 1, 2, 3, 4, 5 , Dirk Daelemans 6, 7, 8, 9 , Erik De Clercq 6, 7, 8, 9 , Christophe Pannecouque 6, 7, 8, 9 , Peng Zhan 1, 2, 3, 4, 5 , Xinyong Liu 1, 2, 3, 4, 5
Affiliation
|
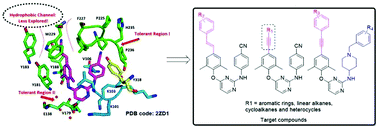
A new series of diarylpyrimidines (DAPYs) were designed, synthesized and evaluated as novel HIV-1 NNRTIs to further explore the chemical space surrounding the “hydrophobic channel” of the NNRTI binding pocket (NNIBP), guided by the comprehensive analysis of X-ray structural biology data of HIV-1 RT/NNRTI complexes and molecular modeling. Encouragingly, most of the synthesized DAPYs were found to be active against the HIV-1 wild-type (WT) strain with EC50 values ranging from 3 nM to 63 nM, and displayed significantly reduced cytotoxicity compared with etravirine (ETV) and rilpivirine (RPV). Among them, two most promising compounds Z10 (EC50 = 3 nM) and Z13 (EC50 = 3 nM) showed equivalent potency against the HIV-1 WT strain to the reference drugs efavirenz (EFV, EC50 = 3 nM) and ETV (EC50 = 3 nM). Notably, Z13 also showed the most potent activity against HIV-1 mutant strains including K103N (EC50 = 10 nM), E138K (EC50 = 22 nM) and RES056 (EC50 = 0.935 μM). Against mutant strains Y181C, Y188L and F227L + V106A, Z17 showed double-digit nanomolar inhibitory activity with EC50 values 27 nM, 98 nM and 30 nM, respectively. The structure–activity relationships (SARs) and molecular docking studies provided important clues for further molecular elaboration. Collectively, this study provides useful information to guide lead optimization and drug discovery via the exploration of this seldom investigated region.
中文翻译:

通过研究鲜为人知的“疏水通道”的化学空间,发现新颖的二芳基嘧啶作为有效的HIV-1 NNRTIs
设计,合成和评估了一系列新的二芳基嘧啶(DAPYs),作为新型HIV-1 NNRTI,在X射线综合分析的指导下,进一步探索了NNRTI结合口袋(NNIBP)“疏水通道”周围的化学空间HIV-1 RT / NNRTI复合物的结构生物学数据和分子模型。令人鼓舞的是,发现大多数合成的DAPY对HIV-1野生型(WT)菌株均具有EC 50值为3 nM至63 nM的活性,并且与依曲韦林(ETV)和利比韦林相比具有显着降低的细胞毒性( RPV)。其中,两种最有前途的化合物Z10(EC 50 = 3 nM)和Z13(EC 50= 3 nM)对HIV-1 WT菌株显示的效价与参比药物依非韦伦(efavirenz)(EFV,EC 50 = 3 nM)和ETV(EC 50 = 3 nM)相当。值得注意的是,Z13还显示出对HIV-1突变株最有效的活性,包括K103N(EC 50 = 10 nM),E138K(EC 50 = 22 nM)和RES056(EC 50 = 0.935μM )。对突变体菌株Y181C,Y188L和F227L + V106A,Z17呈现两位数纳摩尔抑制活性用EC 50值分别为27 nM,98 nM和30 nM。结构-活性关系(SAR)和分子对接研究为进一步的分子加工提供了重要的线索。总的来说,这项研究提供了有用的信息,可通过对这个很少研究的地区进行探索来指导前导优化和药物发现。
更新日期:2017-12-19
中文翻译:

通过研究鲜为人知的“疏水通道”的化学空间,发现新颖的二芳基嘧啶作为有效的HIV-1 NNRTIs
设计,合成和评估了一系列新的二芳基嘧啶(DAPYs),作为新型HIV-1 NNRTI,在X射线综合分析的指导下,进一步探索了NNRTI结合口袋(NNIBP)“疏水通道”周围的化学空间HIV-1 RT / NNRTI复合物的结构生物学数据和分子模型。令人鼓舞的是,发现大多数合成的DAPY对HIV-1野生型(WT)菌株均具有EC 50值为3 nM至63 nM的活性,并且与依曲韦林(ETV)和利比韦林相比具有显着降低的细胞毒性( RPV)。其中,两种最有前途的化合物Z10(EC 50 = 3 nM)和Z13(EC 50= 3 nM)对HIV-1 WT菌株显示的效价与参比药物依非韦伦(efavirenz)(EFV,EC 50 = 3 nM)和ETV(EC 50 = 3 nM)相当。值得注意的是,Z13还显示出对HIV-1突变株最有效的活性,包括K103N(EC 50 = 10 nM),E138K(EC 50 = 22 nM)和RES056(EC 50 = 0.935μM )。对突变体菌株Y181C,Y188L和F227L + V106A,Z17呈现两位数纳摩尔抑制活性用EC 50值分别为27 nM,98 nM和30 nM。结构-活性关系(SAR)和分子对接研究为进一步的分子加工提供了重要的线索。总的来说,这项研究提供了有用的信息,可通过对这个很少研究的地区进行探索来指导前导优化和药物发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号