当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, characterization and cytotoxicity of arene–ruthenium(ii) complexes with acylpyrazolones functionalized with aromatic groups in the acyl moiety†
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7dt04249c Fabio Marchetti 1, 2, 3, 4 , Riccardo Pettinari 2, 3, 4, 5 , Corrado Di Nicola 1, 2, 3, 4 , Claudio Pettinari 2, 3, 4, 5 , Jessica Palmucci 1, 2, 3, 4 , Rosario Scopelliti 6, 7, 8, 9 , Tina Riedel 6, 7, 8, 9 , Bruno Therrien 9, 10, 11, 12 , Agustín Galindo 13, 14, 15, 16, 17 , Paul J. Dyson 6, 7, 8, 9
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7dt04249c Fabio Marchetti 1, 2, 3, 4 , Riccardo Pettinari 2, 3, 4, 5 , Corrado Di Nicola 1, 2, 3, 4 , Claudio Pettinari 2, 3, 4, 5 , Jessica Palmucci 1, 2, 3, 4 , Rosario Scopelliti 6, 7, 8, 9 , Tina Riedel 6, 7, 8, 9 , Bruno Therrien 9, 10, 11, 12 , Agustín Galindo 13, 14, 15, 16, 17 , Paul J. Dyson 6, 7, 8, 9
Affiliation
|
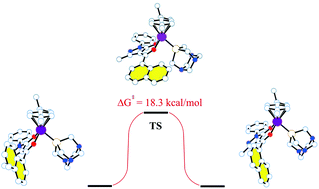
A series of neutral ruthenium(II)–arene complexes, [(arene)Ru(QR)Cl] (arene = p-cymene or hexamethylbenzene), containing 4-acyl-5-pyrazolonate (QR) ligands with aromatic substituents in the acyl moiety (a phenyl in QPh and a 1-naphthyl in Qnaph) and related ionic complexes [(arene)Ru(QR)(PTA)][PF6] (PTA = 1,3,5-triaza-7-phosphaadamantane) have been synthesized and characterized by IR, 1H, 13C and 31P NMR spectroscopy, elemental analysis and ESI mass spectrometry. The structures of five of these compounds were also determined by X-ray crystallography. DFT studies have been performed on all complexes and, in the case of two cationic [(arene)Ru(Qnaph)(PTA)][PF6], the existence of two conformers with a different relative orientation of the naphthyl group in the Qnaph ligand has been assessed, showing that they possess similar energies, in agreement with the experimentally observed NMR spectra in solution. The cytotoxicity of the 4-acyl-5-pyrazolonate proligands (HQR) and complexes was evaluated in vitro against human ovarian carcinoma cells (A2780 and A2780cisR) and non-tumorous human embryonic kidney (HEK293) cells. In general, each complex is about equally cytotoxic to all three cell lines and the PTA derivatives with the naphthyl-modified QR ligands are the most active of the series.
中文翻译:

芳基 -钌(ii)与酰基吡唑啉酮配合物的芳香部分经酰基部分官能化的配合物的合成,表征和细胞毒性†
一系列中性钌(II)-芳烃络合物[[(arene)Ru(Q R)Cl](芳烃=对苯甲基或六甲基苯),在其中含有4-酰基-5-吡唑啉酸酯(Q R)配体,在其中酰基部分(Q Ph中的苯基和Q naph中的1-萘基)和相关的离子络合物[(arene)Ru(Q R)(PTA)] [PF 6 ](PTA = 1,3,5-triaza-已合成并通过IR,1 H,13 C和31表征了7-磷金刚烷)P NMR光谱,元素分析和ESI质谱。这些化合物中的五个的结构也通过X射线晶体学测定。已对所有配合物进行了DFT研究,并且在两个阳离子[[arene)Ru(Q naph)(PTA)] [PF 6 ]的情况下,存在两个构象异构体中萘基的相对取向不同已经评估了Q naph配体,表明它们具有相似的能量,这与在溶液中实验观察到的NMR光谱一致。在体外评估了4-酰基-5-吡唑啉酸酯配体(HQ R)和复合物的细胞毒性抗人卵巢癌细胞(A2780和A2780cisR)和非肿瘤人胚胎肾(HEK293)细胞。通常,每种复合物对所有三个细胞系具有相同的细胞毒性,并且具有萘基修饰的Q R配体的PTA衍生物是该系列中最活跃的。
更新日期:2017-12-19
中文翻译:

芳基 -钌(ii)与酰基吡唑啉酮配合物的芳香部分经酰基部分官能化的配合物的合成,表征和细胞毒性†
一系列中性钌(II)-芳烃络合物[[(arene)Ru(Q R)Cl](芳烃=对苯甲基或六甲基苯),在其中含有4-酰基-5-吡唑啉酸酯(Q R)配体,在其中酰基部分(Q Ph中的苯基和Q naph中的1-萘基)和相关的离子络合物[(arene)Ru(Q R)(PTA)] [PF 6 ](PTA = 1,3,5-triaza-已合成并通过IR,1 H,13 C和31表征了7-磷金刚烷)P NMR光谱,元素分析和ESI质谱。这些化合物中的五个的结构也通过X射线晶体学测定。已对所有配合物进行了DFT研究,并且在两个阳离子[[arene)Ru(Q naph)(PTA)] [PF 6 ]的情况下,存在两个构象异构体中萘基的相对取向不同已经评估了Q naph配体,表明它们具有相似的能量,这与在溶液中实验观察到的NMR光谱一致。在体外评估了4-酰基-5-吡唑啉酸酯配体(HQ R)和复合物的细胞毒性抗人卵巢癌细胞(A2780和A2780cisR)和非肿瘤人胚胎肾(HEK293)细胞。通常,每种复合物对所有三个细胞系具有相同的细胞毒性,并且具有萘基修饰的Q R配体的PTA衍生物是该系列中最活跃的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号