当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting exciton coupling of ligand radical intervalence charge transfer transitions to tune NIR absorption†
Chemical Science ( IF 7.6 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7sc04537a Ryan M Clarke 1 , Tiffany Jeen 1 , Serena Rigo 1 , John R Thompson 1 , Loren G Kaake 1 , Fabrice Thomas 2 , Tim Storr 1
Chemical Science ( IF 7.6 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7sc04537a Ryan M Clarke 1 , Tiffany Jeen 1 , Serena Rigo 1 , John R Thompson 1 , Loren G Kaake 1 , Fabrice Thomas 2 , Tim Storr 1
Affiliation
|
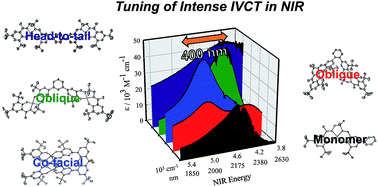
We detail the rational design of a series of bimetallic bis-ligand radical Ni salen complexes in which the relative orientation of the ligand radical chromophores provides a mechanism to tune the energy of intense intervalence charge transfer (IVCT) bands in the near infrared (NIR) region. Through a suite of experimental (electrochemistry, electron paramagnetic resonance spectroscopy, UV-vis-NIR spectroscopy) and theoretical (density functional theory) techniques, we demonstrate that bimetallic Ni salen complexes form bis-ligand radicals upon two-electron oxidation, whose NIR absorption energies depend on the geometry imposed in the bis-ligand radical complex. Relative to the oxidized monomer [1˙]+ (E = 4500 cm−1, ε = 27 700 M−1 cm−1), oxidation of the cofacially constrained analogue 2 to [2˙˙]2+ results in a blue-shifted NIR band (E = 4830 cm−1, ε = 42 900 M−1 cm−1), while oxidation of 5 to [5˙˙]2+, with parallel arrangement of chromophores, results in a red-shifted NIR band (E = 4150 cm−1, ε = 46 600 M−1 cm−1); the NIR bands exhibit double the intensity in comparison to the monomer. Oxidation of the intermediate orientations results in band splitting for [3˙˙]2+ (E = 4890 and 4200 cm−1; ε = 26 500 and 21 100 M−1 cm−1), and a red-shift for [4˙˙]2+ using ortho- and meta-phenylene linkers, respectively. This study demonstrates for the first time, the applicability of exciton coupling to ligand radical systems absorbing in the NIR region and shows that by simple geometry changes, it is possible to tune the energy of intense low energy absorption by nearly 400 nm.
中文翻译:

利用配体自由基间隔电荷转移跃迁的激子耦合来调节近红外吸收†
我们详细介绍了一系列双金属双配体自由基 Ni salen 配合物的合理设计,其中配体自由基发色团的相对方向提供了一种调节近红外 (NIR) 中强间隔电荷转移 (IVCT) 谱带能量的机制。地区。通过一系列实验(电化学、电子顺磁共振光谱、紫外-可见-近红外光谱)和理论(密度泛函理论)技术,我们证明双金属Ni salen配合物在双电子氧化时形成双配体自由基,其近红外吸收能量取决于双配体自由基络合物中施加的几何形状。相对于氧化单体 [ 1˙ ] + ( E = 4500 cm −1 , ε = 27 700 M −1 cm −1 ),共面约束类似物2氧化为 [ 2˙˙ ] 2+会产生蓝色-近红外波段( E = 4830 cm −1 , ε = 42 900 M −1 cm −1 )发生偏移,而5氧化为 [ 5˙˙ ] 2+ ,发色团平行排列,导致近红外波段红移( E = 4150 cm -1 , ε = 46 600 M -1 cm -1 );与单体相比,近红外波段的强度增加了一倍。 中间取向的氧化导致 [ 3˙˙ ] 2+ ( E = 4890 和 4200 cm −1 ; ε = 26 500 和 21 100 M −1 cm −1 ) 的能带分裂,以及 [ 4 的红移˙˙ ] 2+分别使用邻位和间位亚苯基连接体。这项研究首次证明了激子耦合对近红外区域吸收的配体自由基系统的适用性,并表明通过简单的几何变化,可以将强烈的低能量吸收的能量调节近 400 nm。
更新日期:2017-12-19
中文翻译:

利用配体自由基间隔电荷转移跃迁的激子耦合来调节近红外吸收†
我们详细介绍了一系列双金属双配体自由基 Ni salen 配合物的合理设计,其中配体自由基发色团的相对方向提供了一种调节近红外 (NIR) 中强间隔电荷转移 (IVCT) 谱带能量的机制。地区。通过一系列实验(电化学、电子顺磁共振光谱、紫外-可见-近红外光谱)和理论(密度泛函理论)技术,我们证明双金属Ni salen配合物在双电子氧化时形成双配体自由基,其近红外吸收能量取决于双配体自由基络合物中施加的几何形状。相对于氧化单体 [ 1˙ ] + ( E = 4500 cm −1 , ε = 27 700 M −1 cm −1 ),共面约束类似物2氧化为 [ 2˙˙ ] 2+会产生蓝色-近红外波段( E = 4830 cm −1 , ε = 42 900 M −1 cm −1 )发生偏移,而5氧化为 [ 5˙˙ ] 2+ ,发色团平行排列,导致近红外波段红移( E = 4150 cm -1 , ε = 46 600 M -1 cm -1 );与单体相比,近红外波段的强度增加了一倍。 中间取向的氧化导致 [ 3˙˙ ] 2+ ( E = 4890 和 4200 cm −1 ; ε = 26 500 和 21 100 M −1 cm −1 ) 的能带分裂,以及 [ 4 的红移˙˙ ] 2+分别使用邻位和间位亚苯基连接体。这项研究首次证明了激子耦合对近红外区域吸收的配体自由基系统的适用性,并表明通过简单的几何变化,可以将强烈的低能量吸收的能量调节近 400 nm。











































 京公网安备 11010802027423号
京公网安备 11010802027423号