当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed Evolution of an Artificial Imine Reductase
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201711016 Martina Hestericová 1 , Tillman Heinisch 1 , Lur Alonso-Cotchico 2 , Jean-Didier Maréchal 2 , Pietro Vidossich 2 , Thomas R. Ward 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201711016 Martina Hestericová 1 , Tillman Heinisch 1 , Lur Alonso-Cotchico 2 , Jean-Didier Maréchal 2 , Pietro Vidossich 2 , Thomas R. Ward 1
Affiliation
|
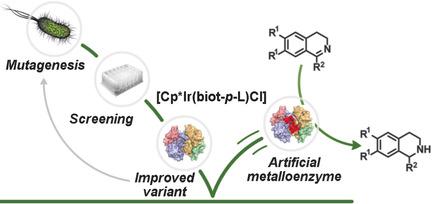
Artificial metalloenzymes, resulting from incorporation of a metal cofactor within a host protein, have received increasing attention in the last decade. The directed evolution is presented of an artificial transfer hydrogenase (ATHase) based on the biotin‐streptavidin technology using a straightforward procedure allowing screening in cell‐free extracts. Two streptavidin isoforms were yielded with improved catalytic activity and selectivity for the reduction of cyclic imines. The evolved ATHases were stable under biphasic catalytic conditions. The X‐ray structure analysis reveals that introducing bulky residues within the active site results in flexibility changes of the cofactor, thus increasing exposure of the metal to the protein surface and leading to a reversal of enantioselectivity. This hypothesis was confirmed by a multiscale approach based mostly on molecular dynamics and protein–ligand dockings.
中文翻译:

人工亚胺还原酶的定向进化
在过去的十年中,由于金属辅酶掺入宿主蛋白而产生的人工金属酶已受到越来越多的关注。提出了一种基于生物素-链霉亲和素技术的人工转移氢化酶(ATHase)的定向进化方法,该方法可用于筛选无细胞提取物中的简单方法。产生了两种链霉亲和素同工型,具有改进的催化活性和对减少环亚胺的选择性。进化的ATH酶在双相催化条件下是稳定的。X射线结构分析表明,在活性位点引入大的残基会导致辅因子的柔性变化,从而增加金属对蛋白质表面的暴露并导致对映选择性的逆转。
更新日期:2018-01-16
中文翻译:

人工亚胺还原酶的定向进化
在过去的十年中,由于金属辅酶掺入宿主蛋白而产生的人工金属酶已受到越来越多的关注。提出了一种基于生物素-链霉亲和素技术的人工转移氢化酶(ATHase)的定向进化方法,该方法可用于筛选无细胞提取物中的简单方法。产生了两种链霉亲和素同工型,具有改进的催化活性和对减少环亚胺的选择性。进化的ATH酶在双相催化条件下是稳定的。X射线结构分析表明,在活性位点引入大的残基会导致辅因子的柔性变化,从而增加金属对蛋白质表面的暴露并导致对映选择性的逆转。











































 京公网安备 11010802027423号
京公网安备 11010802027423号