当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using Mg‐Al Mixed Oxide and Reconstructed Hydrotalcite as Basic Catalysts for Aldol Condensation of Furfural and Cyclohexanone
ChemCatChem ( IF 3.8 ) Pub Date : 2018-02-19 , DOI: 10.1002/cctc.201701880 Oleg Kikhtyanin 1, 2 , David Kadlec 1 , Romana Velvarská 1 , David Kubička 2
ChemCatChem ( IF 3.8 ) Pub Date : 2018-02-19 , DOI: 10.1002/cctc.201701880 Oleg Kikhtyanin 1, 2 , David Kadlec 1 , Romana Velvarská 1 , David Kubička 2
Affiliation
|
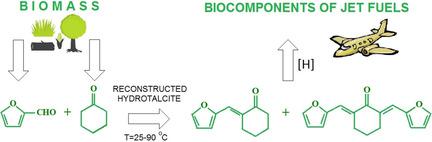
This study presents results on aldol condensation of furfural and cyclohexanone in presence of Mg‐Al hydrotalcite‐derived materials as solid basic catalysts at reaction temperatures from 25 to 90 °C and a cyclohexanone to furfural molar ratio of 1–10. Mg‐Al mixed oxide exhibited reasonable activity with furfural conversion of ca. 50 % after 180 min of the reaction at T=90 °C. The activity of reconstructed hydrotalcite was much higher with furfural conversion close to 100 % at short reaction times. In comparison with Mg‐Al mixed oxide, the initial reaction rate has increased 30–50 times. Under similar reaction conditions, cyclohexanone self‐condensation on HTC‐derived catalysts could not compete with aldol condensation because the former reaction was inhibited by produced water. The change in CH/F molar ratio influenced both furfural conversion and product selectivity; higher furfural content in the reaction mixture favored the second condensation step.
中文翻译:

使用Mg-Al混合氧化物和重组水滑石为基础的糠醛和环己酮醛醇缩合催化剂
这项研究给出了在Mg-Al水滑石衍生的材料作为固体碱性催化剂存在下,糠醛和环己酮的醛醇缩合反应的结果,其反应温度为25至90°C,环己酮与糠醛的摩尔比为1-10。Mg-Al混合氧化物具有约糠醛转化率的合理活性。在T反应180分钟后达到50%= 90℃。在较短的反应时间内,糠醛转化率接近100%,重建的水滑石的活性更高。与Mg-Al混合氧化物相比,初始反应速率提高了30–50倍。在相似的反应条件下,HTC衍生的催化剂上的环己酮自缩合不能与醛醇缩合竞争,因为前者的反应被采出水抑制了。CH / F摩尔比的变化影响糠醛转化率和产物选择性。反应混合物中较高的糠醛含量有利于第二缩合步骤。
更新日期:2018-02-19
中文翻译:

使用Mg-Al混合氧化物和重组水滑石为基础的糠醛和环己酮醛醇缩合催化剂
这项研究给出了在Mg-Al水滑石衍生的材料作为固体碱性催化剂存在下,糠醛和环己酮的醛醇缩合反应的结果,其反应温度为25至90°C,环己酮与糠醛的摩尔比为1-10。Mg-Al混合氧化物具有约糠醛转化率的合理活性。在T反应180分钟后达到50%= 90℃。在较短的反应时间内,糠醛转化率接近100%,重建的水滑石的活性更高。与Mg-Al混合氧化物相比,初始反应速率提高了30–50倍。在相似的反应条件下,HTC衍生的催化剂上的环己酮自缩合不能与醛醇缩合竞争,因为前者的反应被采出水抑制了。CH / F摩尔比的变化影响糠醛转化率和产物选择性。反应混合物中较高的糠醛含量有利于第二缩合步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号