当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structures of the Peptidoglycan N-Acetylglucosamine Deacetylase Bc1974 and Its Complexes with Zinc Metalloenzyme Inhibitors
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.biochem.7b00919 Petros Giastas 1, 2 , Athena Andreou 1 , Athanasios Papakyriakou 1, 3 , Dimitris Koutsioulis 4 , Stavroula Balomenou 4, 5 , Socrates J. Tzartos 2 , Vassilis Bouriotis 4, 5 , Elias E. Eliopoulos 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.biochem.7b00919 Petros Giastas 1, 2 , Athena Andreou 1 , Athanasios Papakyriakou 1, 3 , Dimitris Koutsioulis 4 , Stavroula Balomenou 4, 5 , Socrates J. Tzartos 2 , Vassilis Bouriotis 4, 5 , Elias E. Eliopoulos 1
Affiliation
|
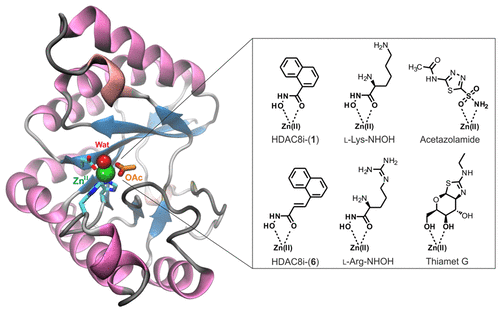
The cell wall peptidoglycan is recognized as a primary target of the innate immune system, and usually its disintegration results in bacterial lysis. Bacillus cereus, a close relative of the highly virulent Bacillus anthracis, contains 10 polysaccharide deacetylases. Among these, the peptidoglycan N-acetylglucosamine deacetylase Bc1974 is the highest homologue to the Bacillus anthracis Ba1977 that is required for full virulence and is involved in resistance to the host’s lysozyme. These metalloenzymes belong to the carbohydrate esterase family 4 (CE4) and are attractive targets for the development of new anti-infective agents. Herein we report the first X-ray crystal structures of the NodB domain of Bc1974, the conserved catalytic core of CE4s, in the unliganded form and in complex with four known metalloenzyme inhibitors and two amino acid hydroxamates that target the active site metal. These structures revealed the presence of two conformational states of a catalytic loop known as motif-4 (MT4), which were not observed previously for peptidoglycan deacetylases, but were recently shown in the structure of a Vibrio clolerae chitin deacetylase. By employing molecular docking of a substrate model, we describe a catalytic mechanism that probably involves initial binding of the substrate in a receptive, more open state of MT4 and optimal catalytic activity in the closed state of MT4, consistent with the previous observations. The ligand-bound structures presented here, in addition to the five Bc1974 inhibitors identified, provide a valuable basis for the design of antibacterial agents that target the peptidoglycan deacetylase Ba1977.
中文翻译:

肽聚糖N-乙酰氨基葡萄糖脱乙酰基酶Bc1974的结构及其与锌金属酶抑制剂的配合物
细胞壁肽聚糖被认为是先天免疫系统的主要靶标,通常其分解会导致细菌裂解。蜡状芽孢杆菌(Bacillus cereus)是高毒性炭疽芽孢杆菌的近亲,含有10种多糖脱乙酰酶。其中,肽聚糖N-乙酰氨基葡糖脱乙酰酶Bc1974是炭疽芽孢杆菌的最高同源物充分毒力所必需的Ba1977,并参与了对宿主溶菌酶的抗性。这些金属酶属于碳水化合物酯酶家族4(CE4),是开发新型抗感染剂的有吸引力的目标。在这里,我们报告的Bc1974 NodB域的第一个X射线晶体结构,CE4s的保守催化核心,以未配体形式并与四种已知的金属酶抑制剂和两种靶向活性位点金属的氨基酸异羟肟酸酯复合。这些结构揭示了被称为基序4(MT4)的催化环的两个构象状态的存在,以前对于肽聚糖脱乙酰基酶没有观察到,但最近在克氏弧菌的结构中显示出了这种构象状态。几丁质脱乙酰基酶。通过使用底物模型的分子对接,我们描述了一种催化机制,该机制可能涉及底物在MT4的接受性,更开放状态下的初始结合以及在MT4闭合状态下的最佳催化活性,这与先前的观察结果一致。除了鉴定出的五种Bc1974抑制剂外,本文介绍的与配体结合的结构为靶向肽聚糖脱乙酰基酶Ba1977的抗菌剂的设计提供了有价值的基础。
更新日期:2018-01-05
中文翻译:

肽聚糖N-乙酰氨基葡萄糖脱乙酰基酶Bc1974的结构及其与锌金属酶抑制剂的配合物
细胞壁肽聚糖被认为是先天免疫系统的主要靶标,通常其分解会导致细菌裂解。蜡状芽孢杆菌(Bacillus cereus)是高毒性炭疽芽孢杆菌的近亲,含有10种多糖脱乙酰酶。其中,肽聚糖N-乙酰氨基葡糖脱乙酰酶Bc1974是炭疽芽孢杆菌的最高同源物充分毒力所必需的Ba1977,并参与了对宿主溶菌酶的抗性。这些金属酶属于碳水化合物酯酶家族4(CE4),是开发新型抗感染剂的有吸引力的目标。在这里,我们报告的Bc1974 NodB域的第一个X射线晶体结构,CE4s的保守催化核心,以未配体形式并与四种已知的金属酶抑制剂和两种靶向活性位点金属的氨基酸异羟肟酸酯复合。这些结构揭示了被称为基序4(MT4)的催化环的两个构象状态的存在,以前对于肽聚糖脱乙酰基酶没有观察到,但最近在克氏弧菌的结构中显示出了这种构象状态。几丁质脱乙酰基酶。通过使用底物模型的分子对接,我们描述了一种催化机制,该机制可能涉及底物在MT4的接受性,更开放状态下的初始结合以及在MT4闭合状态下的最佳催化活性,这与先前的观察结果一致。除了鉴定出的五种Bc1974抑制剂外,本文介绍的与配体结合的结构为靶向肽聚糖脱乙酰基酶Ba1977的抗菌剂的设计提供了有价值的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号