当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Energetics Underlying Twist Polymorphisms in Amyloid Fibrils
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.jpcb.7b10233 Xavier Periole 1 , Thomas Huber 2 , Alessandra Bonito-Oliva 2 , Karina C Aberg 2 , Patrick C A van der Wel 3 , Thomas P Sakmar 2, 4 , Siewert J Marrink 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.jpcb.7b10233 Xavier Periole 1 , Thomas Huber 2 , Alessandra Bonito-Oliva 2 , Karina C Aberg 2 , Patrick C A van der Wel 3 , Thomas P Sakmar 2, 4 , Siewert J Marrink 1
Affiliation
|
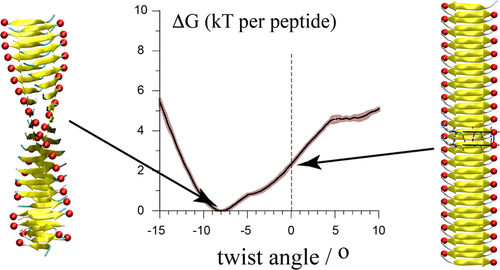
Amyloid fibrils are highly ordered protein aggregates associated with more than 40 human diseases. The exact conditions under which the fibrils are grown determine many types of reported fibril polymorphism, including different twist patterns. Twist-based polymorphs display unique mechanical properties in vitro, and the relevance of twist polymorphism in amyloid diseases has been suggested. We present transmission electron microscopy images of Aβ42-derived (amyloid β) fibrils, which are associated with Alzheimer’s disease, demonstrating the presence of twist variability even within a single long fibril. To better understand the molecular underpinnings of twist polymorphism, we present a structural and thermodynamics analysis of molecular dynamics simulations of the twisting of β-sheet protofilaments of a well-characterized cross-β model: the GNNQQNY peptide from the yeast prion Sup35. The results show that a protofilament model of GNNQQNY is able to adopt twist angles from −11° on the left-hand side to +8° on the right-hand side in response to various external conditions, keeping an unchanged peptide structure. The potential of mean force (PMF) of this cross-β structure upon twisting revealed that only ∼2kBT per peptide are needed to stabilize a straight conformation with respect to the left-handed free-energy minimum. The PMF also shows that the canonical structural core of β-sheets, i.e., the hydrogen-bonded backbone β-strands, favors the straight conformation. However, the concerted effects of the side chains contribute to twisting, which provides a rationale to correlate polypeptide sequence, environmental growth conditions and number of protofilaments in a fibril with twist polymorphisms.
中文翻译:

淀粉样原纤维扭曲多态性背后的能量学
淀粉样原纤维是高度有序的蛋白质聚集体,与 40 多种人类疾病相关。原纤维生长的确切条件决定了许多已报道的原纤维多态性类型,包括不同的扭曲模式。基于扭曲的多晶型物在体外表现出独特的机械特性,并且已经提出扭曲多态性与淀粉样蛋白疾病的相关性。我们展示了 Aβ42 衍生的(β 淀粉样蛋白)原纤维的透射电子显微镜图像,这些原纤维与阿尔茨海默病相关,证明即使在单个长原纤维内也存在扭曲变异性。为了更好地理解扭曲多态性的分子基础,我们对一个充分表征的交叉β模型的β-折叠原丝的扭曲的分子动力学模拟进行了结构和热力学分析:来自酵母朊病毒Sup35的GNNQQNY肽。结果表明,GNNQQNY的原丝模型能够响应各种外部条件而采用从左侧-11°到右侧+8°的扭转角度,保持肽结构不变。这种交叉β结构在扭曲时的平均力(PMF)潜力表明,每个肽只需要~2 k B T就可以稳定相对于左手自由能最小值的直构象。 PMF还表明,β-折叠的规范结构核心,即氢键主链β-链,有利于直构象。然而,侧链的协同作用有助于扭曲,这提供了将多肽序列、环境生长条件和具有扭曲多态性的原纤维中的原丝数量相关联的基本原理。
更新日期:2018-01-05
中文翻译:

淀粉样原纤维扭曲多态性背后的能量学
淀粉样原纤维是高度有序的蛋白质聚集体,与 40 多种人类疾病相关。原纤维生长的确切条件决定了许多已报道的原纤维多态性类型,包括不同的扭曲模式。基于扭曲的多晶型物在体外表现出独特的机械特性,并且已经提出扭曲多态性与淀粉样蛋白疾病的相关性。我们展示了 Aβ42 衍生的(β 淀粉样蛋白)原纤维的透射电子显微镜图像,这些原纤维与阿尔茨海默病相关,证明即使在单个长原纤维内也存在扭曲变异性。为了更好地理解扭曲多态性的分子基础,我们对一个充分表征的交叉β模型的β-折叠原丝的扭曲的分子动力学模拟进行了结构和热力学分析:来自酵母朊病毒Sup35的GNNQQNY肽。结果表明,GNNQQNY的原丝模型能够响应各种外部条件而采用从左侧-11°到右侧+8°的扭转角度,保持肽结构不变。这种交叉β结构在扭曲时的平均力(PMF)潜力表明,每个肽只需要~2 k B T就可以稳定相对于左手自由能最小值的直构象。 PMF还表明,β-折叠的规范结构核心,即氢键主链β-链,有利于直构象。然而,侧链的协同作用有助于扭曲,这提供了将多肽序列、环境生长条件和具有扭曲多态性的原纤维中的原丝数量相关联的基本原理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号