当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed CO-free cyclizative carbonylation of 2-benzylpyridines leading to pyridoisoquinolinones†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7qo01081h Rui Yang 1, 2, 3, 4, 5 , Jin-Tao Yu 1, 2, 3, 4, 5 , Song Sun 1, 2, 3, 4, 5 , Jiang Cheng 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1039/c7qo01081h Rui Yang 1, 2, 3, 4, 5 , Jin-Tao Yu 1, 2, 3, 4, 5 , Song Sun 1, 2, 3, 4, 5 , Jiang Cheng 1, 2, 3, 4, 5
Affiliation
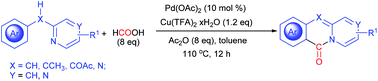
|
A CO-free palladium-catalyzed cyclizative carbonylation of 2-benzylpyridines was developed, leading to pyridoisoquinolinones. This procedure proceeded with the sequential carbonylation of the ortho-C–H bond and the dearomative C–N bond formation. The combination of acetic anhydride and formic acid rather than toxic CO gas was employed in this reaction. The ortho-C–H palladation intermediate palladacycle, which showed comparable activity during this reaction, was well characterized. Notably, N-phenyl-2-pyridinamine also worked well to provide 11H-pyrido[2,1-b]quinazolin-11-one.
中文翻译:

钯催化的2-苄基吡啶的无CO环化羰基化反应生成吡啶基异喹啉酮†
开发了一种无CO的2-苄基吡啶钯催化环化羰基化反应,从而生成吡啶基异喹啉酮。该过程先进行邻-C-H键的顺序羰基化和脱芳香性C-N键的形成。在该反应中使用乙酸酐和甲酸的组合而不是有毒的CO气体。很好地表征了在此反应过程中表现出可比活性的邻-C–H palpalation中间体帕拉达环。值得注意的是,N-苯基-2-吡啶胺也很好地提供了11 H-吡啶并[ 2,1- b ]喹唑啉-11-一。
更新日期:2017-12-19
中文翻译:

钯催化的2-苄基吡啶的无CO环化羰基化反应生成吡啶基异喹啉酮†
开发了一种无CO的2-苄基吡啶钯催化环化羰基化反应,从而生成吡啶基异喹啉酮。该过程先进行邻-C-H键的顺序羰基化和脱芳香性C-N键的形成。在该反应中使用乙酸酐和甲酸的组合而不是有毒的CO气体。很好地表征了在此反应过程中表现出可比活性的邻-C–H palpalation中间体帕拉达环。值得注意的是,N-苯基-2-吡啶胺也很好地提供了11 H-吡啶并[ 2,1- b ]喹唑啉-11-一。



























 京公网安备 11010802027423号
京公网安备 11010802027423号