当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Is there computational support for an unprotonated carbon in the E4 state of nitrogenase?
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-12-18 , DOI: 10.1002/jcc.25145 Per E. M. Siegbahn 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-12-18 , DOI: 10.1002/jcc.25145 Per E. M. Siegbahn 1
Affiliation
|
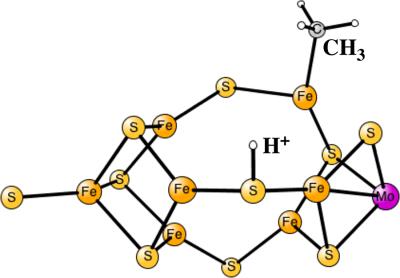
In the key enzyme for nitrogen fixation in nature, nitrogenase, the active site has a metal cluster with seven irons and one molybdenum bound by bridging sulfurs. Surprisingly, there is also a carbon in the center of the cluster, with a role that is not known. A mechanism has been suggested experimentally, where two hydrides leave as a hydrogen molecule in the critical E4 state. A structure with two hydrides, two protonated sulfurs and an unprotonated carbon has been suggested for this state. Rather recently, DFT calculations supported the experimental mechanism but found an active state where the central carbon is protonated all the way to CH3. Even more recently, another DFT study was made that instead supported the experimentally suggested structure. To sort out the origin of these quite different computational results, additional calculations have here been performed using different DFT functionals. The conclusion from these calculations is very clear and shows no computational support for an unprotonated carbon in E4. © 2017 Wiley Periodicals, Inc.
中文翻译:

在固氮酶的 E4 状态下是否有非质子化碳的计算支持?
在自然界中固氮的关键酶固氮酶中,活性位点有一个金属簇,其中有 7 个铁和 1 个钼通过桥接硫键结合。令人惊讶的是,在簇的中心也有一个碳,其作用未知。实验提出了一种机制,其中两个氢化物在临界 E4 状态下作为氢分子离开。对于这种状态,已经提出了具有两个氢化物、两个质子化硫和一个未质子化碳的结构。最近,DFT 计算支持了实验机制,但发现了一种活性状态,其中中心碳一直质子化为 CH3。最近,进行了另一项 DFT 研究,以支持实验建议的结构。为了理清这些截然不同的计算结果的由来,这里使用不同的 DFT 函数进行了额外的计算。这些计算得出的结论非常清楚,并且没有显示对 E4 中未质子化碳的计算支持。© 2017 威利期刊公司。
更新日期:2017-12-18
中文翻译:

在固氮酶的 E4 状态下是否有非质子化碳的计算支持?
在自然界中固氮的关键酶固氮酶中,活性位点有一个金属簇,其中有 7 个铁和 1 个钼通过桥接硫键结合。令人惊讶的是,在簇的中心也有一个碳,其作用未知。实验提出了一种机制,其中两个氢化物在临界 E4 状态下作为氢分子离开。对于这种状态,已经提出了具有两个氢化物、两个质子化硫和一个未质子化碳的结构。最近,DFT 计算支持了实验机制,但发现了一种活性状态,其中中心碳一直质子化为 CH3。最近,进行了另一项 DFT 研究,以支持实验建议的结构。为了理清这些截然不同的计算结果的由来,这里使用不同的 DFT 函数进行了额外的计算。这些计算得出的结论非常清楚,并且没有显示对 E4 中未质子化碳的计算支持。© 2017 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号