当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heat Shock Proteins Revisited: Using a Mutasynthetically Generated Reblastatin Library to Compare the Inhibition of Human and Leishmania Hsp90s
ChemBioChem ( IF 2.6 ) Pub Date : 2018-02-19 , DOI: 10.1002/cbic.201700616 Sona Mohammadi-Ostad-Kalayeh 1 , Frank Stahl 2 , Thomas Scheper 2 , Klaus Kock 3 , Christian Herrmann 3 , Fernanda Aparecida Heleno Batista 4 , Júlio César Borges 4 , Florenz Sasse 5 , Simone Eichner 6 , Jekaterina Ongouta 6 , Carsten Zeilinger 1 , Andreas Kirschning 6
ChemBioChem ( IF 2.6 ) Pub Date : 2018-02-19 , DOI: 10.1002/cbic.201700616 Sona Mohammadi-Ostad-Kalayeh 1 , Frank Stahl 2 , Thomas Scheper 2 , Klaus Kock 3 , Christian Herrmann 3 , Fernanda Aparecida Heleno Batista 4 , Júlio César Borges 4 , Florenz Sasse 5 , Simone Eichner 6 , Jekaterina Ongouta 6 , Carsten Zeilinger 1 , Andreas Kirschning 6
Affiliation
|
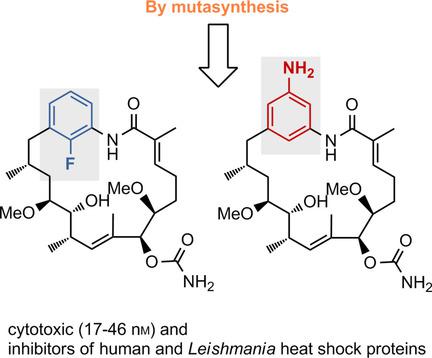
Heat shock protein (Hsp) inhibition in parasites: New reblastatin derivatives, prepared by mutasynthesis complemented with a library of other Hsp inhibitors, were evaluated in cell‐based and in in vitro ATP displacement assays, the latter utilising purified human HsHsp90 and LbHsp90 from L. braziliensis. The study paves the way for finding selective inhibitors of parasitic heat shock proteins.

中文翻译:

再次探讨热休克蛋白:使用多基因合成的雷抑素文库来比较人类和利什曼原虫Hsp90s的抑制作用
寄生虫中的热休克蛋白(Hsp)抑制作用:通过诱变合成与其他Hsp抑制剂文库互补的新再生素衍生物,已在基于细胞的体外ATP置换测定中进行了评估,后者利用L纯化的人HsHsp90和LbHsp90 。braziliensis。该研究为寻找寄生性热激蛋白的选择性抑制剂铺平了道路。

更新日期:2018-02-19

中文翻译:

再次探讨热休克蛋白:使用多基因合成的雷抑素文库来比较人类和利什曼原虫Hsp90s的抑制作用
寄生虫中的热休克蛋白(Hsp)抑制作用:通过诱变合成与其他Hsp抑制剂文库互补的新再生素衍生物,已在基于细胞的体外ATP置换测定中进行了评估,后者利用L纯化的人HsHsp90和LbHsp90 。braziliensis。该研究为寻找寄生性热激蛋白的选择性抑制剂铺平了道路。










































 京公网安备 11010802027423号
京公网安备 11010802027423号