当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Regio‐ and Stereodivergent Access to 1,2‐Amino Alcohols or 1,4‐Fluoro Alcohols by NHC‐Catalyzed Ring Opening of Epoxy enals
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201709823 Si Bei Poh 1 , Jun-Yang Ong 1 , Shenci Lu 1 , Yu Zhao 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201709823 Si Bei Poh 1 , Jun-Yang Ong 1 , Shenci Lu 1 , Yu Zhao 1
Affiliation
|
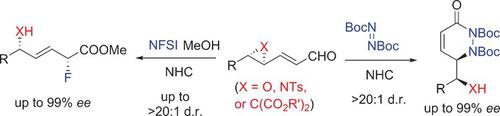
Described is an unprecedented NHC‐catalyzed (NHC=N‐heterocyclic carbene), stereoselective ring opening of epoxy and cyclopropyl enals to deliver valuable compounds bearing multiple stereocenters. A straightforward three‐step procedure involving two catalytic enantioselective transformations has been developed and leads to a regio‐ and stereodivergent synthesis of either 1,2‐amino alcohols/diamines or 1,4‐fluoro alcohols with excellent diastereo‐ and enantiopurity.
中文翻译:

NHC催化的环氧环戊烯开环可高度区域和立体分散地获得1,2-氨基醇或1,4-氟醇
描述了前所未有的NHC催化(NHC = N-杂环卡宾),环氧和环丙基烯醛的立体选择性开环,以提供带有多个立体中心的有价值的化合物。已经开发了一种简单的三步方法,涉及两个催化对映选择性转化,并导致区域和立体发散合成具有出色的非对映和对映纯度的1,2-氨基醇/二胺或1,4-氟醇。
更新日期:2018-01-16
中文翻译:

NHC催化的环氧环戊烯开环可高度区域和立体分散地获得1,2-氨基醇或1,4-氟醇
描述了前所未有的NHC催化(NHC = N-杂环卡宾),环氧和环丙基烯醛的立体选择性开环,以提供带有多个立体中心的有价值的化合物。已经开发了一种简单的三步方法,涉及两个催化对映选择性转化,并导致区域和立体发散合成具有出色的非对映和对映纯度的1,2-氨基醇/二胺或1,4-氟醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号