当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Annulation between Aryl Iodides and Epoxides through Palladium/Norbornene Cooperative Catalysis
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201712393 Renhe Li 1 , Guangbin Dong 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201712393 Renhe Li 1 , Guangbin Dong 1
Affiliation
|
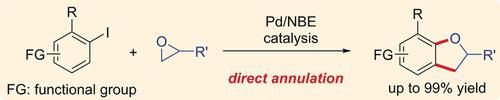
Herein we report a direct annulation between aryl iodides and epoxides through palladium/norbornene (Pd/NBE) cooperative catalysis. An iso‐propyl ester substituted NBE was found to be most efficient to suppress the formation of multiple‐NBE‐insertion byproducts and affords the desired 2,3‐dihydrobenzofuran derivatives in 44–99 % yields. The reaction is scalable and tolerates a range of functional groups. Asymmetric synthesis is realized using an enantiopure epoxide. Application of this method into a concise synthesis of insecticide fufenozide is demonstrated.
中文翻译:

通过钯/降冰片烯协同催化在芳基碘化物和环氧化物之间的直接成环
本文中,我们报告了通过钯/降冰片烯(Pd / NBE)协同催化在芳基碘化物和环氧化物之间的直接环化反应。发现异丙酯取代的NBE最有效地抑制了多次插入NBE的副产物的形成,并以44-99%的收率提供了所需的2,3-二氢苯并呋喃衍生物。该反应是可扩展的,并且可以耐受一定范围的官能团。使用对映纯的环氧化物可实现不对称合成。证明了该方法在杀虫剂夫非尼嗪的简明合成中的应用。
更新日期:2018-01-16
中文翻译:

通过钯/降冰片烯协同催化在芳基碘化物和环氧化物之间的直接成环
本文中,我们报告了通过钯/降冰片烯(Pd / NBE)协同催化在芳基碘化物和环氧化物之间的直接环化反应。发现异丙酯取代的NBE最有效地抑制了多次插入NBE的副产物的形成,并以44-99%的收率提供了所需的2,3-二氢苯并呋喃衍生物。该反应是可扩展的,并且可以耐受一定范围的官能团。使用对映纯的环氧化物可实现不对称合成。证明了该方法在杀虫剂夫非尼嗪的简明合成中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号