当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring ECD on a Benchtop Q Exactive Orbitrap Mass Spectrometer
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.jproteome.7b00622 Kyle L. Fort 1, 2 , Christian N. Cramer 1, 3, 4 , Valery G. Voinov 5, 6 , Yury V. Vasil’ev 5, 6 , Nathan I. Lopez 5 , Joseph S. Beckman 5, 6 , Albert J. R. Heck 1, 2
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.jproteome.7b00622 Kyle L. Fort 1, 2 , Christian N. Cramer 1, 3, 4 , Valery G. Voinov 5, 6 , Yury V. Vasil’ev 5, 6 , Nathan I. Lopez 5 , Joseph S. Beckman 5, 6 , Albert J. R. Heck 1, 2
Affiliation
|
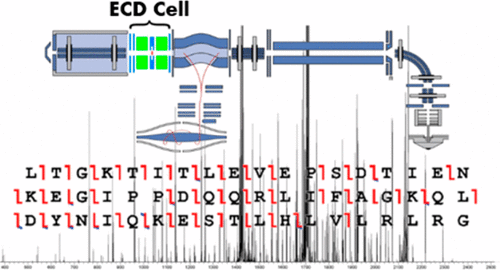
As the application of mass spectrometry intensifies in scope and diversity, the need for advanced instrumentation addressing a wide variety of analytical needs also increases. To this end, many modern, top-end mass spectrometers are designed or modified to include a wider range of fragmentation technologies, for example, ECD, ETD, EThcD, and UVPD. Still, the majority of instrument platforms are limited to more conventional methods, such as CID and HCD. While these latter methods have performed well, the less conventional fragmentation methods have been shown to lead to increased information in many applications including middle-down proteomics, top-down proteomics, glycoproteomics, and disulfide bond mapping. We describe the modification of the popular Q Exactive Orbitrap mass spectrometer to extend its fragmentation capabilities to include ECD. We show that this modification allows ≥85% matched ion intensity to originate from ECD fragment ion types as well as provides high sequence coverage (≥60%) of intact proteins and high fragment identification rates with ∼70% of ion signals matched. Finally, the ECD implementation promotes selective disulfide bond dissociation, facilitating the identification of disulfide-linked peptide conjugates. Collectively, this modification extends the capabilities of the Q Exactive Orbitrap mass spectrometer to a range of new applications.
中文翻译:

在台式Q有源Orbitrap质谱仪上探索ECD
随着质谱技术的应用范围和多样性的增强,对满足多种分析需求的先进仪器的需求也在增加。为此,对许多现代高端质谱仪进行了设计或修改,使其包括更广泛的碎片化技术,例如ECD,ETD,EThcD和UVPD。尽管如此,大多数仪器平台仍限于更常规的方法,例如CID和HCD。尽管后一种方法表现良好,但已显示出较不常规的片段化方法可在许多应用中增加信息,包括中下蛋白质组学,自上而下蛋白质组学,糖蛋白组学和二硫键图谱分析。我们描述了对流行的Q Exactive Orbitrap质谱仪的修改,以扩展其碎片化能力以包括ECD。我们表明,这种修饰可以使≥85%的匹配离子强度源自ECD片段离子类型,并提供完整蛋白的高序列覆盖率(≥60%),以及〜70%匹配的离子信号的高片段识别率。最后,ECD的实施促进了选择性的二硫键解离,促进了二硫键连接的肽缀合物的鉴定。总体而言,此修改将Q Exactive Orbitrap质谱仪的功能扩展到了一系列新应用中。有助于鉴定二硫键连接的肽缀合物。总体而言,此修改将Q Exactive Orbitrap质谱仪的功能扩展到了一系列新应用中。有助于鉴定二硫键连接的肽缀合物。总体而言,此修改将Q Exactive Orbitrap质谱仪的功能扩展到了一系列新应用中。
更新日期:2017-12-29
中文翻译:

在台式Q有源Orbitrap质谱仪上探索ECD
随着质谱技术的应用范围和多样性的增强,对满足多种分析需求的先进仪器的需求也在增加。为此,对许多现代高端质谱仪进行了设计或修改,使其包括更广泛的碎片化技术,例如ECD,ETD,EThcD和UVPD。尽管如此,大多数仪器平台仍限于更常规的方法,例如CID和HCD。尽管后一种方法表现良好,但已显示出较不常规的片段化方法可在许多应用中增加信息,包括中下蛋白质组学,自上而下蛋白质组学,糖蛋白组学和二硫键图谱分析。我们描述了对流行的Q Exactive Orbitrap质谱仪的修改,以扩展其碎片化能力以包括ECD。我们表明,这种修饰可以使≥85%的匹配离子强度源自ECD片段离子类型,并提供完整蛋白的高序列覆盖率(≥60%),以及〜70%匹配的离子信号的高片段识别率。最后,ECD的实施促进了选择性的二硫键解离,促进了二硫键连接的肽缀合物的鉴定。总体而言,此修改将Q Exactive Orbitrap质谱仪的功能扩展到了一系列新应用中。有助于鉴定二硫键连接的肽缀合物。总体而言,此修改将Q Exactive Orbitrap质谱仪的功能扩展到了一系列新应用中。有助于鉴定二硫键连接的肽缀合物。总体而言,此修改将Q Exactive Orbitrap质谱仪的功能扩展到了一系列新应用中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号