当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improved Protease-Targeting and Biopharmaceutical Properties of Novel Prodrugs of Ganciclovir
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00792 Kefeng Sun 1 , Hao Xu 2 , John L. Hilfinger 3 , Kyung-Dall Lee 2 , Chester J. Provoda 4 , Hairat Sabit 5 , Gordon L. Amidon 2
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00792 Kefeng Sun 1 , Hao Xu 2 , John L. Hilfinger 3 , Kyung-Dall Lee 2 , Chester J. Provoda 4 , Hairat Sabit 5 , Gordon L. Amidon 2
Affiliation
|
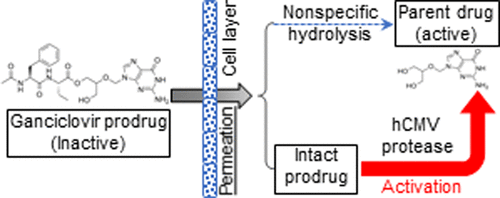
The prodrug strategy has been frequently employed as a chemical approach for overcoming the disadvantages of existing parent drugs. In this report, we synthesized four monoester prodrugs of ganciclovir, an anticytomegalovirus drug, and demonstrated their potential advantages in protease-targeted activation and biopharmaceutical profiles over the parent compound. We demonstrated that these four prodrugs of ganciclovir, i.e., N-benzyloxycarbonyl-(L)-alanine-ganciclovir (CbzAlaGCV), N-benzyloxycarbonyl-(α,l)-aminobutyric acid-ganciclovir (CbzAbuGCV), N-acetyl-(l)-phenylalanine-(l)-alanine-ganciclovir (AcPheAlaGCV), and N-acetyl-(l)-phenylalanine-(α,l)-aminobutyric acid-ganciclovir (AcPheAbuGCV), are hydrolytically activated by the protease of human cytomegalovirus (hCMV), a serine protease that possesses intrinsic esterase activities. CbzAlaGCV and AcPheAlaGCV were found to be activated at a higher rate by the hCMV protease than CbzAbuGCV and AcPheAbuGCV. These ganciclovir prodrugs could potentially be targeted to selective activation by the hCMV protease which is only present at the viral infection sites, thereby achieving higher efficacy and lower systemic toxicity. The tissue stability, cellular uptake, and trans-epithelial transport of these ganciclovir prodrugs were also characterized. The N-acetylated dipeptide prodrugs of ganciclovir were found to be generally more stable than Cbz-amino acid prodrugs in various tissue matrices. Among the four prodrug candidates, AcPheAbuGCV was the most stable in human cell homogenates, plasma, and pooled liver microsomes. AcPheAbuGCV also possessed a superior cellular uptake profile and permeability across epithelial cell monolayers. Since the targeting and selective activation of a prodrug is determined by not only its rate of hydrolysis catalyzed by the hCMV protease target but also its biopharmaceutical properties, i.e., oral absorption and systemic availability, AcPheAbuGCV is considered the best overall candidate among the four ganciclovir prodrugs for further research and development for treatment of hCMV infection.
中文翻译:

更昔洛韦新型前药的蛋白酶靶向和生物制药性能的改善
前药策略经常被用作克服现有母体药物缺点的化学方法。在本报告中,我们合成了更昔洛韦(一种抗巨细胞病毒药物)的四种单酯前药,并证明了它们在蛋白酶靶向活化和生物制药方面比母体化合物潜在的优势。我们证明了更昔洛韦的这四种前药,即N-苄氧基羰基-(L)-丙氨酸-更昔洛韦(CbzAlaGCV),N-苄氧基羰基- ((α,l)-氨基丁酸-更昔洛韦(CbzAbuGCV),N-乙酰基-(l)-苯丙氨酸-(l)-丙氨酸-更昔洛韦(AcPheAlaGCV)和N-乙酰基-(l)-苯丙氨酸-(α,1)-氨基丁酸-更昔洛韦(AcPheAbuGCV)被人类巨细胞病毒(hCMV)的蛋白酶水解激活,该蛋白是一种丝氨酸蛋白酶,具有固有的酯酶活性。发现hCMV蛋白酶比CbzAbuGCV和AcPheAbuGCV以更高的速率激活CbzAlaGCV和AcPheAlaGCV。这些更昔洛韦前药可能会被hCMV蛋白酶选择性激活,而hCMV蛋白酶仅存在于病毒感染部位,从而获得更高的功效和更低的全身毒性。还对这些更昔洛韦前药的组织稳定性,细胞摄取和上皮运输进行了表征。该ñ发现更昔洛韦的β-乙酰化二肽前药在各种组织基质中通常比Cbz-氨基酸前药更稳定。在这四种前药候选物中,AcPheAbuGCV在人细胞匀浆,血浆和肝微粒体中最稳定。AcPheAbuGCV还具有优异的细胞摄取特性和跨上皮细胞单层的通透性。由于前体药物的靶向和选择性激活不仅取决于其被hCMV蛋白酶靶标催化的水解速率,还取决于其生物药物特性(即口服吸收和全身可利用性),因此AcPheAbuGCV被认为是四种更昔洛韦前体药物中最佳的整体候选药物。进一步研究和开发用于治疗hCMV感染的药物。
更新日期:2018-01-05
中文翻译:

更昔洛韦新型前药的蛋白酶靶向和生物制药性能的改善
前药策略经常被用作克服现有母体药物缺点的化学方法。在本报告中,我们合成了更昔洛韦(一种抗巨细胞病毒药物)的四种单酯前药,并证明了它们在蛋白酶靶向活化和生物制药方面比母体化合物潜在的优势。我们证明了更昔洛韦的这四种前药,即N-苄氧基羰基-(L)-丙氨酸-更昔洛韦(CbzAlaGCV),N-苄氧基羰基- ((α,l)-氨基丁酸-更昔洛韦(CbzAbuGCV),N-乙酰基-(l)-苯丙氨酸-(l)-丙氨酸-更昔洛韦(AcPheAlaGCV)和N-乙酰基-(l)-苯丙氨酸-(α,1)-氨基丁酸-更昔洛韦(AcPheAbuGCV)被人类巨细胞病毒(hCMV)的蛋白酶水解激活,该蛋白是一种丝氨酸蛋白酶,具有固有的酯酶活性。发现hCMV蛋白酶比CbzAbuGCV和AcPheAbuGCV以更高的速率激活CbzAlaGCV和AcPheAlaGCV。这些更昔洛韦前药可能会被hCMV蛋白酶选择性激活,而hCMV蛋白酶仅存在于病毒感染部位,从而获得更高的功效和更低的全身毒性。还对这些更昔洛韦前药的组织稳定性,细胞摄取和上皮运输进行了表征。该ñ发现更昔洛韦的β-乙酰化二肽前药在各种组织基质中通常比Cbz-氨基酸前药更稳定。在这四种前药候选物中,AcPheAbuGCV在人细胞匀浆,血浆和肝微粒体中最稳定。AcPheAbuGCV还具有优异的细胞摄取特性和跨上皮细胞单层的通透性。由于前体药物的靶向和选择性激活不仅取决于其被hCMV蛋白酶靶标催化的水解速率,还取决于其生物药物特性(即口服吸收和全身可利用性),因此AcPheAbuGCV被认为是四种更昔洛韦前体药物中最佳的整体候选药物。进一步研究和开发用于治疗hCMV感染的药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号