当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Structure Revision of Dichrocephones A and B
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201711766 Volker M. Schmiedel 1 , Young J. Hong 2 , Dieter Lentz 1 , Dean J. Tantillo 2 , Mathias Christmann 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201711766 Volker M. Schmiedel 1 , Young J. Hong 2 , Dieter Lentz 1 , Dean J. Tantillo 2 , Mathias Christmann 1
Affiliation
|
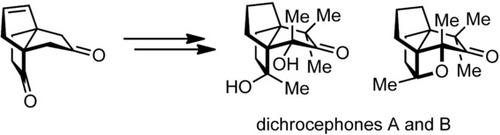
Herein, we report the first enantioselective synthesis of dichrocephones A and B, which are cytotoxic triquinane sesquiterpenes with a dense array of stereogenic centers within a strained polycyclic environment. Key features include the application of a catalytic asymmetric Wittig reaction, followed by stereoselective functionalization of the propellane core into a pentacyclic intermediate. Double reductive ring cleavage yielded the proposed structure of dichrocephone A. Mismatched spectroscopic data for our synthetic material compared to the natural isolate led us to revise the previously proposed configuration based on biosynthetic considerations and NMR calculations. Implementation of these findings culminated in the synthesis of dichrocephones A and B.
中文翻译:

二向声电话A和B的合成和结构修订
在此,我们报告了二苯甲醚A和B的首次对映选择性合成,二苯甲醚是具有毒性的多环环境中密集的立体定位中心的细胞毒性三喹烷倍半萜。关键特征包括应用催化不对称Wittig反应,然后将丙炔核心立体选择性官能化为五环中间体。双还原性环裂解产生了拟二甲醚A的拟议结构。与天然分离物相比,我们的合成材料的光谱数据不匹配,导致我们基于生物合成考量和NMR计算,修改了之前提出的构型。这些发现的实现最终导致了二苯甲醚A和B的合成。
更新日期:2018-01-16
中文翻译:

二向声电话A和B的合成和结构修订
在此,我们报告了二苯甲醚A和B的首次对映选择性合成,二苯甲醚是具有毒性的多环环境中密集的立体定位中心的细胞毒性三喹烷倍半萜。关键特征包括应用催化不对称Wittig反应,然后将丙炔核心立体选择性官能化为五环中间体。双还原性环裂解产生了拟二甲醚A的拟议结构。与天然分离物相比,我们的合成材料的光谱数据不匹配,导致我们基于生物合成考量和NMR计算,修改了之前提出的构型。这些发现的实现最终导致了二苯甲醚A和B的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号