当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of the right-hand cores of 16-methylated oxazolomycins
Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-18 , DOI: 10.1016/j.tet.2017.12.036 Kohei Eto , Jun Ishihara , Susumi Hatakeyama
中文翻译:

立体选择性合成16-甲基化恶唑球霉素的右手核
更新日期:2017-12-18
Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-18 , DOI: 10.1016/j.tet.2017.12.036 Kohei Eto , Jun Ishihara , Susumi Hatakeyama
|
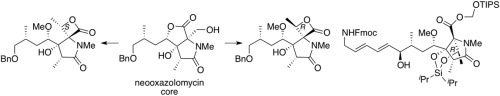
The right-hand heterocyclic cores of oxazolomycins having either 16R or 16S-methyl group configurations on the β-lactones were stereoselectively synthesized from the common intermediate utilized for our previous syntheses of neooxazolomycin and oxazolomycin A. In addition, the right-hand segment required for the synthesis of KSM-2690 and lajollamycin members was also synthesized in a stereoselective manner.
中文翻译:

立体选择性合成16-甲基化恶唑球霉素的右手核
β-内酯上具有16 R或16 S-甲基构型的恶唑菌素的右侧杂环核芯是从我们先前合成新恶唑菌霉素和恶唑菌素A所用的常见中间体中立体选择性合成的。还以立体选择性的方式合成了合成KSM-2690和拉柔霉素成员所需要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号