Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-18 , DOI: 10.1016/j.tet.2017.12.035 Robert Kawęcki , Wojciech Stańczyk , Agnieszka Jaglińska
|
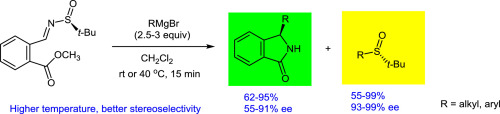
A reaction of Grignard reagents with an optically pure N-sulfinylimine derived from methyl 2-formylbenzoate yields enantioenriched isoindolinones and tert-butyl sulfoxides. The products are formed by the addition of the nucleophile to N-sulfinylimine followed by cyclization to form N-tert-butylsulfinylisoindolinone, which readily undergoes substitution with a second equivalent of Grignard reagent. The reaction can be carried out in dichloromethane at room temperature or at elevated temperatures without any loss of stereoselectivity. The use of nucleophiles other than Grignard reagents has also been investigated.
中文翻译:

立体选择性合成异吲哚啉酮和叔丁基亚砜
格氏试剂与衍生自2-甲酰基苯甲酸甲酯的光学纯的N-亚磺酰亚胺反应,得到对映体富集的异吲哚啉酮和叔丁基亚砜。该产品通过加入亲核试剂,以形成Ñ -sulfinylimine随后环化,以形成ñ -叔-butylsulfinylisoindolinone,这容易经历取代与格氏试剂的第二等效。该反应可以在二氯甲烷中在室温或升高的温度下进行,而没有任何立体选择性的损失。还研究了格氏试剂以外的亲核试剂的使用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号