当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Low Overpotential Electroreduction of CO2 to CO on a Supported Gold Catalyst in an Alkaline Flow Electrolyzer
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acsenergylett.7b01096 Sumit Verma 1, 2 , Yuki Hamasaki 2, 3 , Chaerin Kim 2, 3 , Wenxin Huang 2, 3 , Shawn Lu 1 , Huei-Ru Molly Jhong 1, 2 , Andrew A. Gewirth 2, 4 , Tsuyohiko Fujigaya 2, 3 , Naotoshi Nakashima 2, 3 , Paul J. A. Kenis 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acsenergylett.7b01096 Sumit Verma 1, 2 , Yuki Hamasaki 2, 3 , Chaerin Kim 2, 3 , Wenxin Huang 2, 3 , Shawn Lu 1 , Huei-Ru Molly Jhong 1, 2 , Andrew A. Gewirth 2, 4 , Tsuyohiko Fujigaya 2, 3 , Naotoshi Nakashima 2, 3 , Paul J. A. Kenis 1, 2
Affiliation
|
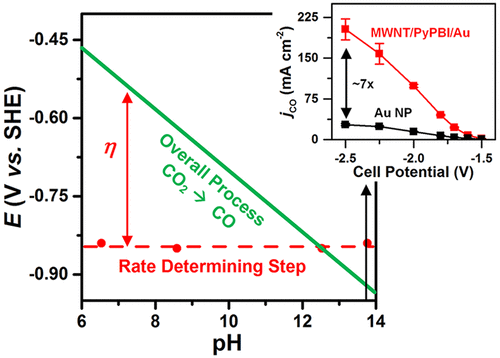
Cost competitive electroreduction of CO2 to CO requires electrochemical systems that exhibit partial current density (jCO) exceeding 150 mA cm–2 at cell overpotentials (|ηcell|) less than 1 V. However, achieving such benchmarks remains difficult. Here, we report the electroreduction of CO2 on a supported gold catalyst in an alkaline flow electrolyzer with performance levels close to the economic viability criteria. Onset of CO production occurred at cell and cathode overpotentials of just −0.25 and −0.02 V, respectively. High jCO (∼99, 158 mA cm–2) was obtained at low |ηcell| (∼0.70, 0.94 V) and high CO energetic efficiency (∼63.8, 49.4%). The performance was stable for at least 8 h. Additionally, the onset cathode potentials, kinetic isotope effect, and Tafel slopes indicate the low overpotential production of CO in alkaline media to be the result of a pH-independent rate-determining step (i.e., electron transfer) in contrast to a pH-dependent overall process.
中文翻译:

碱性流动电解槽中负载型金催化剂上将CO 2低电位电还原为CO的见解
CO的成本竞争力的电还原2至CO需要表现出局部电流密度(电化学系统Ĵ CO)超过150毫安厘米-2在细胞的超电势(|η细胞|)小于1 V.然而,实现这样的基准仍然是困难的。在这里,我们报告了在碱性流电解槽中负载型金催化剂上的CO 2的电还原性能水平接近经济可行性标准。一氧化碳的产生分别发生在电池和阴极的过电势分别为-0.25和-0.02 V的时候。高Ĵ CO(〜99,158毫安厘米-2在低获得)|η细胞| (〜0.70,0.94 V)和高的CO能量效率(〜63.8,49.4%)。性能稳定至少8小时。另外,开始的阴极电势,动力学同位素效应和Tafel斜率表明,在碱性介质中CO低的超电势产生是pH依赖性速率确定步骤(即电子转移)的结果,而不是pH依赖性整个过程。
更新日期:2017-12-18
中文翻译:

碱性流动电解槽中负载型金催化剂上将CO 2低电位电还原为CO的见解
CO的成本竞争力的电还原2至CO需要表现出局部电流密度(电化学系统Ĵ CO)超过150毫安厘米-2在细胞的超电势(|η细胞|)小于1 V.然而,实现这样的基准仍然是困难的。在这里,我们报告了在碱性流电解槽中负载型金催化剂上的CO 2的电还原性能水平接近经济可行性标准。一氧化碳的产生分别发生在电池和阴极的过电势分别为-0.25和-0.02 V的时候。高Ĵ CO(〜99,158毫安厘米-2在低获得)|η细胞| (〜0.70,0.94 V)和高的CO能量效率(〜63.8,49.4%)。性能稳定至少8小时。另外,开始的阴极电势,动力学同位素效应和Tafel斜率表明,在碱性介质中CO低的超电势产生是pH依赖性速率确定步骤(即电子转移)的结果,而不是pH依赖性整个过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号