当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unique Spectroscopic Properties of the H-Cluster in a Putative Sensory [FeFe] Hydrogenase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-09 , DOI: 10.1021/jacs.7b11287 Nipa Chongdar 1 , James A. Birrell 1 , Krzysztof Pawlak 1 , Constanze Sommer 1 , Edward J. Reijerse 1 , Olaf Rüdiger 1 , Wolfgang Lubitz 1 , Hideaki Ogata 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-09 , DOI: 10.1021/jacs.7b11287 Nipa Chongdar 1 , James A. Birrell 1 , Krzysztof Pawlak 1 , Constanze Sommer 1 , Edward J. Reijerse 1 , Olaf Rüdiger 1 , Wolfgang Lubitz 1 , Hideaki Ogata 1, 2
Affiliation
|
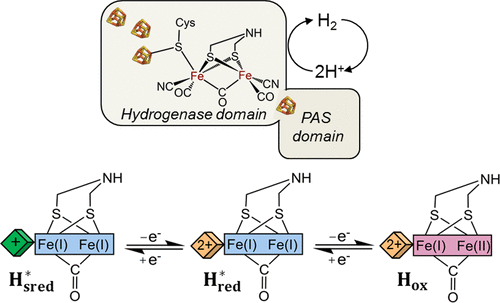
Sensory type [FeFe] hydrogenases are predicted to play a role in transcriptional regulation by detecting the H2 level of the cellular environment. These hydrogenases contain the hydrogenase domain with distinct modifications in the active site pocket, followed by a Per-Arnt-Sim (PAS) domain. As yet, neither the physiological function nor the biochemical or spectroscopic properties of these enzymes have been explored. Here, we present the characterization of an artificially maturated, putative sensory [FeFe] hydrogenase from Thermotoga maritima (HydS). This enzyme shows lower hydrogen conversion activity than prototypical [FeFe] hydrogenases and a reduced inhibition by CO. Using FTIR spectroelectrochemistry and EPR spectroscopy, three redox states of the active site were identified. The spectroscopic signatures of the most oxidized state closely resemble those of the Hox state from the prototypical [FeFe] hydrogenases, while the FTIR spectra of both singly and doubly reduced states show large differences. The FTIR bands of both the reduced states are strongly red-shifted relative to the Hox state, indicating reduction at the diiron site, but with retention of the bridging CO ligand. The unique functional and spectroscopic features of HydS are discussed with regard to the possible role of altered amino acid residues influencing the electronic properties of the H-cluster.
中文翻译:

假定的感官 [FeFe] 氢化酶中 H 簇的独特光谱特性
通过检测细胞环境的 H2 水平,预计感觉型 [FeFe] 氢化酶在转录调控中发挥作用。这些氢化酶在活性位点口袋中包含具有不同修饰的氢化酶结构域,然后是 Per-Arnt-Sim (PAS) 结构域。迄今为止,尚未探索这些酶的生理功能、生化或光谱特性。在这里,我们展示了来自海栖热袍菌 (HydS) 的人工成熟的、假定的感官 [FeFe] 氢化酶的表征。该酶显示出比原型 [FeFe] 氢化酶更低的氢转化活性,并且减少了 CO 的抑制。使用 FTIR 光谱电化学和 EPR 光谱,鉴定了活性位点的三种氧化还原状态。最氧化态的光谱特征与原型 [FeFe] 氢化酶的 Hox 态的光谱特征非常相似,而单还原态和双还原态的 FTIR 光谱显示出很大差异。两种还原态的 FTIR 谱带都相对于 Hox 态发生了强烈的红移,表明在二铁位点还原,但保留了桥接 CO 配体。讨论了 HydS 独特的功能和光谱特征,讨论了改变的氨基酸残基可能影响 H 簇电子特性的作用。表明在二铁位点还原,但保留了桥接 CO 配体。讨论了 HydS 独特的功能和光谱特征,讨论了改变的氨基酸残基可能影响 H 簇电子特性的作用。表明在二铁位点还原,但保留了桥接 CO 配体。讨论了 HydS 独特的功能和光谱特征,讨论了改变的氨基酸残基可能影响 H 簇电子特性的作用。
更新日期:2018-01-09
中文翻译:

假定的感官 [FeFe] 氢化酶中 H 簇的独特光谱特性
通过检测细胞环境的 H2 水平,预计感觉型 [FeFe] 氢化酶在转录调控中发挥作用。这些氢化酶在活性位点口袋中包含具有不同修饰的氢化酶结构域,然后是 Per-Arnt-Sim (PAS) 结构域。迄今为止,尚未探索这些酶的生理功能、生化或光谱特性。在这里,我们展示了来自海栖热袍菌 (HydS) 的人工成熟的、假定的感官 [FeFe] 氢化酶的表征。该酶显示出比原型 [FeFe] 氢化酶更低的氢转化活性,并且减少了 CO 的抑制。使用 FTIR 光谱电化学和 EPR 光谱,鉴定了活性位点的三种氧化还原状态。最氧化态的光谱特征与原型 [FeFe] 氢化酶的 Hox 态的光谱特征非常相似,而单还原态和双还原态的 FTIR 光谱显示出很大差异。两种还原态的 FTIR 谱带都相对于 Hox 态发生了强烈的红移,表明在二铁位点还原,但保留了桥接 CO 配体。讨论了 HydS 独特的功能和光谱特征,讨论了改变的氨基酸残基可能影响 H 簇电子特性的作用。表明在二铁位点还原,但保留了桥接 CO 配体。讨论了 HydS 独特的功能和光谱特征,讨论了改变的氨基酸残基可能影响 H 簇电子特性的作用。表明在二铁位点还原,但保留了桥接 CO 配体。讨论了 HydS 独特的功能和光谱特征,讨论了改变的氨基酸残基可能影响 H 簇电子特性的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号