当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of a nonhydrolyzable nucleotide phosphoroimidazolide analogue that catalyzes nonenzymatic RNA primer extension
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-02 , DOI: 10.1021/jacs.7b11623 Chun Pong Tam 1, 2 , Lijun Zhou 1, 3 , Albert C. Fahrenbach 1, 3, 4 , Wen Zhang 1, 3 , Travis Walton 1, 5 , Jack W. Szostak 1, 2, 3, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-02 , DOI: 10.1021/jacs.7b11623 Chun Pong Tam 1, 2 , Lijun Zhou 1, 3 , Albert C. Fahrenbach 1, 3, 4 , Wen Zhang 1, 3 , Travis Walton 1, 5 , Jack W. Szostak 1, 2, 3, 4
Affiliation
|
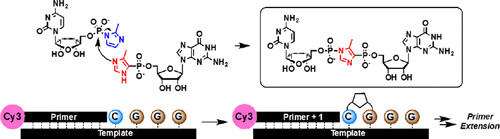
We report the synthesis of guanosine 5′-(4-methylimidazolyl)phosphonate (ICG), the third member of a series of nonhydrolyzable nucleoside 5′-phosphoro-2-methylimidazolide (2-MeImpN) analogues designed for mechanistic studies of nonenzymatic RNA primer extension. The addition of a 2-MeImpN monomer to a primer is catalyzed by the presence of a downstream activated monomer, yet the three nonhydrolyzable analogues do not show catalytic effects under standard mildly basic primer extension conditions. Surprisingly, ICG, which has a pKa similar to that of 2-MeImpG, is a modest catalyst of nonenzymatic primer extension at acidic pH. Here we show that ICG reacts with 2-MeImpC to form a stable 5′–5′-imidazole-bridged guanosine-cytosine dinucleotide, with both a labile nitrogen–phosphorus and a stable carbon–phosphorus linkage flanking the central imidazole bridge. Cognate RNA primer–template complexes react with this GC-dinucleotide by attack of the primer 3′-hydroxyl on the activated N–P side of the 5′-5′-imidazole bridge. These observations support the hypothesis that 5′–5′-imidazole-bridged dinucleotides can bind to cognate RNA primer–template duplexes and adopt appropriate conformations for subsequent phosphodiester bond formation, consistent with our recent mechanistic proposal that the formation of activated 5′–5′-imidazolium-bridged dinucleotides is responsible for 2-MeImpN-driven primer extension.
中文翻译:

催化非酶促 RNA 引物延伸的不可水解核苷酸磷咪唑啉类似物的合成
我们报告了鸟苷 5'-(4-甲基咪唑基)膦酸酯(ICG)的合成,这是一系列不可水解的核苷 5'-磷酸-2-甲基咪唑化物(2-MeImpN)类似物的第三个成员,设计用于非酶 RNA 引物的机械研究扩大。将 2-MeImpN 单体添加到引物是由下游活化单体的存在催化的,但三种不可水解的类似物在标准的温和碱性引物延伸条件下不显示催化作用。令人惊讶的是,ICG 具有与 2-MeImpG 相似的 pKa,是酸性 pH 下非酶促引物延伸的适度催化剂。在这里,我们表明 ICG 与 2-MeImpC 反应形成稳定的 5'-5'-咪唑桥连鸟苷-胞嘧啶二核苷酸,在中央咪唑桥两侧有一个不稳定的氮-磷和一个稳定的碳-磷键。同源 RNA 引物-模板复合物通过攻击 5'-5'-咪唑桥活化 N-P 侧的引物 3'-羟基与该 GC-二核苷酸反应。这些观察结果支持这样的假设,即 5'-5'-咪唑桥接二核苷酸可以与同源 RNA 引物-模板双链体结合,并为随后的磷酸二酯键形成采用适当的构象,这与我们最近的机制提议一致,即活化 5'-5 的形成'-咪唑鎓桥接二核苷酸负责 2-MeImpN 驱动的引物延伸。
更新日期:2018-01-02
中文翻译:

催化非酶促 RNA 引物延伸的不可水解核苷酸磷咪唑啉类似物的合成
我们报告了鸟苷 5'-(4-甲基咪唑基)膦酸酯(ICG)的合成,这是一系列不可水解的核苷 5'-磷酸-2-甲基咪唑化物(2-MeImpN)类似物的第三个成员,设计用于非酶 RNA 引物的机械研究扩大。将 2-MeImpN 单体添加到引物是由下游活化单体的存在催化的,但三种不可水解的类似物在标准的温和碱性引物延伸条件下不显示催化作用。令人惊讶的是,ICG 具有与 2-MeImpG 相似的 pKa,是酸性 pH 下非酶促引物延伸的适度催化剂。在这里,我们表明 ICG 与 2-MeImpC 反应形成稳定的 5'-5'-咪唑桥连鸟苷-胞嘧啶二核苷酸,在中央咪唑桥两侧有一个不稳定的氮-磷和一个稳定的碳-磷键。同源 RNA 引物-模板复合物通过攻击 5'-5'-咪唑桥活化 N-P 侧的引物 3'-羟基与该 GC-二核苷酸反应。这些观察结果支持这样的假设,即 5'-5'-咪唑桥接二核苷酸可以与同源 RNA 引物-模板双链体结合,并为随后的磷酸二酯键形成采用适当的构象,这与我们最近的机制提议一致,即活化 5'-5 的形成'-咪唑鎓桥接二核苷酸负责 2-MeImpN 驱动的引物延伸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号