当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extended Investigation of Electrochemical CO2 Reduction in Ethanolamine Solutions by SECM
Electroanalysis ( IF 2.7 ) Pub Date : 2017-12-18 , DOI: 10.1002/elan.201700693 Daniel Filotás 1, 2 , Tibor Nagy 3 , Livia Nagy 1, 2 , Peter Mizsey 3, 4 , Geza Nagy 1, 2
Electroanalysis ( IF 2.7 ) Pub Date : 2017-12-18 , DOI: 10.1002/elan.201700693 Daniel Filotás 1, 2 , Tibor Nagy 3 , Livia Nagy 1, 2 , Peter Mizsey 3, 4 , Geza Nagy 1, 2
Affiliation
|
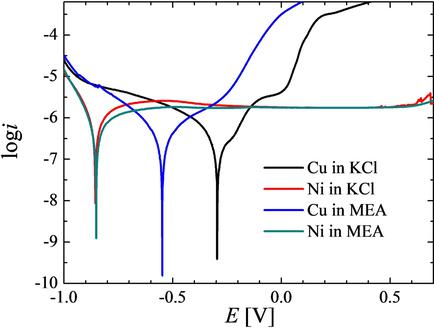
The electrochemical reduction of carbon dioxide is very much in the focus of interest today. Intensive research is carried out in leading laboratories trying to work out methods for making useful materials from this unwanted greenhouse gas using solar or wind power generated excess electric energy. In this work, electrochemical reduction experiments are carried out in homemade cells supplied with different metal electrodes. Electrolytes containing carbon dioxide absorbing components like monoethanolamine (MEA) or KHCO3, KOH, and K2CO3 solutions are used. Metal‐containing species were noticed in the used electrolytes after being in contact with the metal working electrodes. Therefore parallel to the electrochemical measurements, the metal components in the electrolyte were checked with atomic absorption methods for getting better insight into the nature of the electrode passivation. This paper attempts to compare the behavior of different electrode materials (copper, nickel) in CO2 capturing media, and investigate of the products of the electrolysis using Scanning Electrochemical Microscopy (SECM), Atomic Absorption Spectroscopy (AAS) and gas chromatography.
中文翻译:

SECM对乙醇胺溶液中电化学还原CO2的扩展研究
当今,人们非常关注二氧化碳的电化学还原。在领先的实验室中进行了深入的研究,试图找到使用太阳能或风能产生的多余电能从这种不需要的温室气体中制备有用材料的方法。在这项工作中,电化学还原实验是在配有不同金属电极的自制电池中进行的。含有二氧化碳吸收成分的电解质,例如单乙醇胺(MEA)或KHCO 3,KOH和K 2 CO 3使用解决方案。与金属工作电极接触后,在用过的电解液中发现了含金属的物质。因此,与电化学测量平行,用原子吸收法检查了电解质中的金属成分,以更好地了解电极钝化的性质。本文试图比较不同电极材料(铜,镍)在CO 2捕获介质中的行为,并使用扫描电化学显微镜(SECM),原子吸收光谱法(AAS)和气相色谱法研究电解的产物。
更新日期:2017-12-18
中文翻译:

SECM对乙醇胺溶液中电化学还原CO2的扩展研究
当今,人们非常关注二氧化碳的电化学还原。在领先的实验室中进行了深入的研究,试图找到使用太阳能或风能产生的多余电能从这种不需要的温室气体中制备有用材料的方法。在这项工作中,电化学还原实验是在配有不同金属电极的自制电池中进行的。含有二氧化碳吸收成分的电解质,例如单乙醇胺(MEA)或KHCO 3,KOH和K 2 CO 3使用解决方案。与金属工作电极接触后,在用过的电解液中发现了含金属的物质。因此,与电化学测量平行,用原子吸收法检查了电解质中的金属成分,以更好地了解电极钝化的性质。本文试图比较不同电极材料(铜,镍)在CO 2捕获介质中的行为,并使用扫描电化学显微镜(SECM),原子吸收光谱法(AAS)和气相色谱法研究电解的产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号