当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Guiding a divergent reaction by photochemical control: bichromatic selective access to levulinates and butenolides†
Chemical Science ( IF 7.6 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1039/c7sc05094a Revannath L Sutar 1, 2 , Saumik Sen 3 , Or Eivgi 1 , Gal Segalovich 1 , Igor Schapiro 3 , Ofer Reany 2 , N Gabriel Lemcoff 1, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1039/c7sc05094a Revannath L Sutar 1, 2 , Saumik Sen 3 , Or Eivgi 1 , Gal Segalovich 1 , Igor Schapiro 3 , Ofer Reany 2 , N Gabriel Lemcoff 1, 4
Affiliation
|
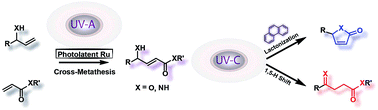
Allylic and acrylic substrates may be efficiently transformed by a sequential bichromatic photochemical process into derivatives of levulinates or butenolides with high selectivity when phenanthrene is used as a regulator. Thus, UV-A photoinduced cross-metathesis (CM) couples the acrylic and allylic counterparts and subsequent UV-C irradiation initiates E–Z isomerization of the carbon–carbon double bond, followed by one of two competing processes; namely, cyclization by transesterification or a 1,5-H shift and tautomerization. Quantum chemical calculations demonstrate that intermediates are strongly blue-shifted for the cyclization while red-shifted for the 1,5-H shift reaction. Hence, delaying the double bond migration by employing UV-C absorbing phenanthrene, results in a selective novel divergent all-photochemical pathway for the synthesis of fundamental structural motifs of ubiquitous natural products.
中文翻译:

通过光化学控制引导发散反应:双色选择性获得乙酰丙酸盐和丁烯内酯†
当菲用作调节剂时,烯丙基和丙烯酸底物可以通过连续的双色光化学过程有效地转化为高选择性的乙酰丙酸酯或丁烯内酯的衍生物。因此,UV-A 光诱导交叉复分解 (CM) 偶联丙烯酸和烯丙基对应物,随后的 UV-C 照射引发碳碳双键的E - Z异构化,随后发生两个竞争过程之一;即,通过酯交换或1,5-H转移和互变异构化进行环化。量子化学计算表明,环化反应的中间体发生强烈蓝移,而 1,5-H 位移反应则发生红移。因此,通过采用 UV-C 吸收菲来延迟双键迁移,产生了一种选择性的新型发散全光化学途径,用于合成普遍存在的天然产物的基本结构基序。
更新日期:2017-12-18
中文翻译:

通过光化学控制引导发散反应:双色选择性获得乙酰丙酸盐和丁烯内酯†
当菲用作调节剂时,烯丙基和丙烯酸底物可以通过连续的双色光化学过程有效地转化为高选择性的乙酰丙酸酯或丁烯内酯的衍生物。因此,UV-A 光诱导交叉复分解 (CM) 偶联丙烯酸和烯丙基对应物,随后的 UV-C 照射引发碳碳双键的E - Z异构化,随后发生两个竞争过程之一;即,通过酯交换或1,5-H转移和互变异构化进行环化。量子化学计算表明,环化反应的中间体发生强烈蓝移,而 1,5-H 位移反应则发生红移。因此,通过采用 UV-C 吸收菲来延迟双键迁移,产生了一种选择性的新型发散全光化学途径,用于合成普遍存在的天然产物的基本结构基序。









































 京公网安备 11010802027423号
京公网安备 11010802027423号