Science Bulletin ( IF 18.8 ) Pub Date : 2017-12-16 , DOI: 10.1016/j.scib.2017.12.013 Hong Wang 1 , Wenjie He 1 , Xing'an Dong 1 , Haiqiang Wang 2 , Fan Dong 1
|
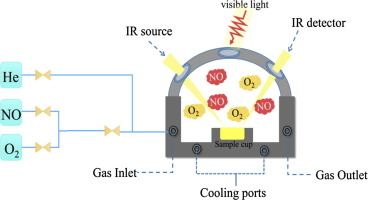
The g-C3N4 with different structures was prepared by heat treatment using urea (CN-U) and thiourea (CN-T) as precursors under the same conditions. The microstructure and optical properties of the photocatalyst were analyzed with advanced tools. The results showed that the CN-U has a porous structure, a high specific surface area and a wide band gap in comparison with CN-T. The in situ FT-IR technique was used to monitor the adsorption and reaction process of visible photocatalytic NO oxidation on g-C3N4. The corresponding reaction mechanism was proposed based on the results of reaction intermediate observation and electron paramagnetic resonance (EPR) radical scavenging. It was revealed that (1) the presence of defective sites favored the adsorption of gas molecules and electronically compensated it leading to promoted formation of the final products; (2) the high separation efficiency of photogenerated electron-hole pairs enhanced the production of radicals during the photocatalytic reaction; (3) the hydroxyl radicals (OH) are not selective for the decomposition of pollutants, which are favorable to the complete oxidation of the reaction intermediates. The above three aspects are the main reasons for the CN-U possessing the efficient visible light photocatalytic activity. The present work could provide new insights and methods for understanding the mechanism of photocatalysis.
中文翻译:

缺陷型 g-C3N4 可见光光催化 NO 氧化反应机理的原位 FT-IR 研究
以尿素(CN-U)和硫脲(CN-T)为前驱体,在相同条件下通过热处理制备了不同结构的gC 3 N 4 。使用先进的工具分析了光催化剂的微观结构和光学性质。结果表明,与CN-T相比,CN-U具有多孔结构、高比表面积和宽禁带。采用原位FT-IR技术监测可见光催化NO氧化在gC 3 N 4上的吸附和反应过程. 基于反应中间体观察和电子顺磁共振(EPR)自由基清除的结果,提出了相应的反应机理。结果表明:(1)缺陷位点的存在有利于气体分子的吸附并对其进行电子补偿,从而促进最终产物的形成;(2) 光生电子-空穴对的高分离效率促进了光催化反应过程中自由基的产生;(3)羟基自由基(OH)对污染物的分解没有选择性,有利于反应中间体的完全氧化。以上三个方面是CN-U具有高效可见光光催化活性的主要原因。目前的工作可以为理解光催化机理提供新的见解和方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号