当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the σ, π and δ hole interactions: a molecular orbital overview†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1039/c7nj03632a V. Angarov 1, 2, 3, 4 , S. Kozuch 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1039/c7nj03632a V. Angarov 1, 2, 3, 4 , S. Kozuch 1, 2, 3, 4
Affiliation
|
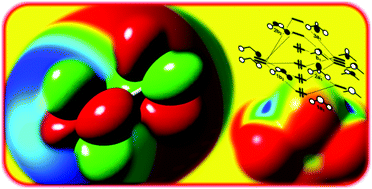
By means of molecular orbital theory and the analysis of charge transfer and electrostatic forces, we discuss the features of non-covalent hole interactions of the halogen, chalcogen and pnictogen bond families. The use of MOs allows us to explain and predict the location of holes, and to design novel interactions such as systems with σ and π holes on the same or opposite sides. In view of the orbital origin of the hole interactions, we suggest that many chalcogen and pnictogen bonds are largely based on π holes and not on the commonly accepted σ holes. In addition, a new type of hole interaction based on δ holes is found on the sextuply bonded dimolybdenum.
中文翻译:

关于σ,π和δ空穴相互作用:分子轨道概览†
通过分子轨道理论以及电荷转移和静电力的分析,我们讨论了卤素,硫属元素和光生原键族的非共价空穴相互作用的特征。MO的使用使我们能够解释和预测孔的位置,并设计新颖的相互作用,例如在相同或相对侧上具有σ和π孔的系统。考虑到空穴相互作用的轨道原点,我们建议许多硫属元素和光成原键主要基于π空穴,而不是基于公认的σ空穴。此外,在六重结合的二钼上发现了一种基于δ空穴的新型空穴相互作用。
更新日期:2017-12-18
中文翻译:

关于σ,π和δ空穴相互作用:分子轨道概览†
通过分子轨道理论以及电荷转移和静电力的分析,我们讨论了卤素,硫属元素和光生原键族的非共价空穴相互作用的特征。MO的使用使我们能够解释和预测孔的位置,并设计新颖的相互作用,例如在相同或相对侧上具有σ和π孔的系统。考虑到空穴相互作用的轨道原点,我们建议许多硫属元素和光成原键主要基于π空穴,而不是基于公认的σ空穴。此外,在六重结合的二钼上发现了一种基于δ空穴的新型空穴相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号